Systems analysis of a RIG-I agonist inducing broad spectrum inhibition of virus infectivity - PubMed (original) (raw)
doi: 10.1371/journal.ppat.1003298. Epub 2013 Apr 25.
David Olagnier, Zhengyun Xu, Suzanne Paz, S Mehdi Belgnaoui, Erin I Lafferty, Valérie Janelle, Meztli Arguello, Marilene Paquet, Khader Ghneim, Stephanie Richards, Andrew Smith, Peter Wilkinson, Mark Cameron, Ulrich Kalinke, Salman Qureshi, Alain Lamarre, Elias K Haddad, Rafick Pierre Sekaly, Suraj Peri, Siddharth Balachandran, Rongtuan Lin, John Hiscott
Affiliations
- PMID: 23633948
- PMCID: PMC3635991
- DOI: 10.1371/journal.ppat.1003298
Systems analysis of a RIG-I agonist inducing broad spectrum inhibition of virus infectivity
Marie-Line Goulet et al. PLoS Pathog. 2013.
Abstract
The RIG-I like receptor pathway is stimulated during RNA virus infection by interaction between cytosolic RIG-I and viral RNA structures that contain short hairpin dsRNA and 5' triphosphate (5'ppp) terminal structure. In the present study, an RNA agonist of RIG-I was synthesized in vitro and shown to stimulate RIG-I-dependent antiviral responses at concentrations in the picomolar range. In human lung epithelial A549 cells, 5'pppRNA specifically stimulated multiple parameters of the innate antiviral response, including IRF3, IRF7 and STAT1 activation, and induction of inflammatory and interferon stimulated genes - hallmarks of a fully functional antiviral response. Evaluation of the magnitude and duration of gene expression by transcriptional profiling identified a robust, sustained and diversified antiviral and inflammatory response characterized by enhanced pathogen recognition and interferon (IFN) signaling. Bioinformatics analysis further identified a transcriptional signature uniquely induced by 5'pppRNA, and not by IFNα-2b, that included a constellation of IRF7 and NF-kB target genes capable of mobilizing multiple arms of the innate and adaptive immune response. Treatment of primary PBMCs or lung epithelial A549 cells with 5'pppRNA provided significant protection against a spectrum of RNA and DNA viruses. In C57Bl/6 mice, intravenous administration of 5'pppRNA protected animals from a lethal challenge with H1N1 Influenza, reduced virus titers in mouse lungs and protected animals from virus-induced pneumonia. Strikingly, the RIG-I-specific transcriptional response afforded partial protection from influenza challenge, even in the absence of type I interferon signaling. This systems approach provides transcriptional, biochemical, and in vivo analysis of the antiviral efficacy of 5'pppRNA and highlights the therapeutic potential associated with the use of RIG-I agonists as broad spectrum antiviral agents.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
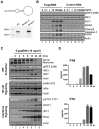
Figure 1. 5′pppRNA stimulates an antiviral and inflammatory response in lung epithelial A549 cells.
(A) Schematic representation of VSV-derived 5′pppRNA and gel analysis. The 5′ppp-containing 67-mer RNA oligonucleotide is derived from the untranslated regions (UTRs) of VSV and the product of in vitro transcription runs as a single product degraded by RNase I. (B) 5′pppRNA or a homologous control RNA lacking a 5′-triphosphate end was mixed with Lipofectamine RNAiMax and transfected at different RNA concentrations (0.1–500 ng/ml) into A549 cells. At 8 h post treatment, whole cell extracts (WCEs) were prepared, resolved by SDS-page and analyzed by immunoblotting for IRF3 pSer396, IRF3, ISG56, NOXA, cleaved caspase 3, PARP and β-actin. Results are from a representative experiment; all immunoblots are from the same samples. (C) A549 cells were transfected with 10 ng/ml 5′pppRNA and WCEs were prepared at different times after transfection (0–48 h), subjected to SDS-PAGE and probed with antibodies for IRF3 pSer-396, IRF3, IRF7, STAT1 pTyr-701, STAT1, ISG56, RIG-I, IκBα pSer-32, IkBα and β-actin; all immunoblots are from the same samples. To detect IRF3 dimerization, WCEs were resolved by native-PAGE and analyzed by immunoblotting for IRF3. (D) ELISA was performed on cell culture supernatants to quantify the release of IFNβ and IFNα over time. Error bars represent SEM from two independent samples.
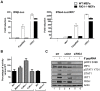
Figure 2. Induction of the interferon response by 5′pppRNA is dependent on functional RIG-I signaling.
(A) WT and RIG-I−/− MEFs were co-transfected with IFNα4 or IFNβ promoter reporter plasmid (200 ng) along with 5′pppRNA (500 ng/ml) or expression plasmids encoding a constitutively active form of RIG-I (ΔRIG-I) (100 ng). IRF-7 expression plasmid (100 ng) was added for transactivation of the IFNα4 promoter. Luciferase activity was analyzed 24 h post-transfection by the Dual-Luciferase Reporter assay. Relative luciferase activity was measured as fold induction relative to the basal level of reporter gene. Error bars represent SEM from nine replicates performed in three independent experiments. (B) Mda5−/−, TLR3−/−, TLR7−/− and RIG-I−/− MEFs were co-transfected with IFNβ promoter reporter plasmid (200 ng) along with 5′pppRNA (500 ng/ml). Luciferase activity was analyzed 24 h post-transfection by the Dual-Luciferase Reporter assay. Relative luciferase activity was measured as fold induction relative to the basal level of reporter gene. Promoter activity in the knockout MEFs was then normalized against the activity in their respective wt MEFs to obtain the percentage of fold activation. Error bars represent SEM from nine replicates performed in three independent experiments. (C) A549 cells were either left untreated or transfected with a control siRNA or RIG-I siRNA. After 48 h, 5′pppRNA (10 ng/mL) was transfected and at 8 h after treatment, WCEs were analyzed by SDS-PAGE and immunoblotted for pIRF3 Ser-396, IRF3, pSTAT1 Tyr 701, STAT1, IFIT1, RIG-I, and β-Actin. Results are from a representative experiment; all immunoblots are from the same samples.
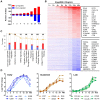
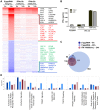
Figure 3. Transcriptome analysis of the host antiviral response to 5′pppRNA.
A549 cells were transfected with 10 ng/ml of 5′pppRNA using Lipofectamine RNAiMax for designated periods of time. Samples were analyzed by Illumina gene expression array and DEG were identified based on fold change ≥±2 and _p_-value ≤0.001 (A) Number of up-regulated and down-regulated DEG at each time point. (B) Heatmap of all DEG sorted by fold change; top 30 genes are listed. Red, Up-regulated; blue, down-regulated. (C) Functional characterization of DEGs following 5′pppRNA treatment based on Ingenuity Pathway Analysis software. Bar height refers to the number of DEG in each pathway and the color refers to the contribution from up-regulated or down-regulated genes. (D) Genes among the top up-regulated genes were selected based on three different expression patterns: early, sustained, late.
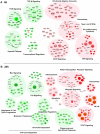
Figure 4. Functional characterization of genes differentially expressed by RIG-I.
The 6 h (A) and 24 h (B) time points from the kinetic analysis were selected to perform a functional classification of the up-regulated (red) and down-regulated (green) genes by 5′pppRNA. Genes with a fold change ≥±2 and _p_-value ≤0.001 were clustered using IPA. The intensity of the color is representative of the fold change. Larger circles indicate a transcription factor.
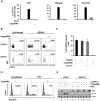
Figure 5. Gene expression profiling of differentially expressed genes in response to 5′pppRNA and IFNα-2b.
A549 cells were transfected with 10 ng/ml of 5′pppRNA using Lipofectamine RNAiMax or treated with IFNα-2b (100 IU/ml or 1000 IU/ml). Samples were collected at 6 h or 24 h post-treatment and were analyzed by Illumina gene expression array. Genes with a fold change ≥±2.0 and _p_-value ≤0.001 were considered differentially expressed. (A) Heatmap showing top DEG affected by 5′pppRNA and IFNα-2b treatments. Genes regulated by both 5′pppRNA and IFNα-2b in at least one condition are indicated in black (up-regulated) or blue (down-regulated). Genes uniquely induced by 5′pppRNA in at least one time point but not by IFNα-2b in any conditions are highlighted in red (up-regulated) or green (down-regulated). Top genes are listed in each instance. (B) Cell culture supernatant was collected at the time of treatment with IFNα-2b (0 h; input), or at 24 h following 5′pppRNA or IFNα-2b treatment and assayed by ELISA for multiple subunits of IFNα. Error bars represent SEM from two independent samples. (C) Surface proportional Venn diagram illustrating the magnitude of the response by 5′pppRNA and IFNα-2b at 6 h and 24 h. Number of DEG is indicated in each area. (D) Comparison of genes induced by each treatment - 5′pppRNA 6 h (dark blue); 5′pppRNA 24 h (light blue); IFNα-2b (1000 IU/ml) 6 h (red); IFNα-2b (1000 IU/ml) 24 h (pink) - based on functional classification by Ingenuity Pathway Analysis.
Figure 6. 5′pppRNA acts as a broad-spectrum antiviral agent.
(A) A549 cells were transfected with 10 ng/ml 5′pppRNA 24 h prior to infection with VSVΔ51-GFP (MOI 0.1), Dengue virus (MOI 0.1), and Vaccinia-GFP virus (MOI 5), respectively. Percentage of infected cells was determined 24 h post-infection by flow cytometry analysis of GFP expression (VSV-GFP and Vaccinia-GFP) or intracellular staining of DENV E protein expression (Dengue virus). Data are from a representative experiment performed in triplicate ± SD. (B) Human PBMCs were transfected with 100 ng/ml 5′pppRNA 24 h prior to infection with dengue virus at an MOI of 5. At 24 h post-infection, the percentage of Dengue infected CD14+ and CD14− cells was evaluated by intracellular staining of DENV E protein expression by flow cytometry. Data are from a representative experiment performed in triplicate ± SD. (C) Human PBMCs from three different donors were transfected with 100 ng/ml 5′pppRNA prior to infection with Dengue virus at an MOI of 5. The percentage of Dengue infected cells in the CD14+ population was evaluated by intracellular staining of DENV E protein expression using flow cytometry. Data are from an experiment performed in triplicate on three different patients ± SD (D) CD4+ T cells isolated from human PBMCs and activated with anti-CD3 and anti-CD28 antibodies. Cells were incubated in the presence or absence of supernatant from 5′pppRNA-treated monocytes for 4 h and infected with HIV-GFP (MOI 0.1) for 48 h. The percentage of HIV infected, activated CD4+ T cells (GFP positive) was assessed by flow cytometry. (E) Huh7 and Huh7.5 were transfected with 5′pppRNA (10 ng/mL) and infected with HCV 24 h later. At 48 h post-infection, WCEs were collected and subjected to SDS PAGE and immunoblot to examine the expression of HCV viral protein NS3, IFIT1, and β-Actin.
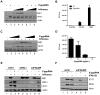
Figure 7. 5′pppRNA inhibits H1N1 Influenza replication in vitro.
(A) A549 cells were treated with 5′pppRNA (10 ng/ml); at 24 h post-treatment, cells were infected with an increasing MOI of A/PR8/34 H1N1 Influenza virus (0.02MOI, 0.2MOI, 2MOI) for 24 h. WCEs were subjected to SDS PAGE and immunoblot to examine the expression of influenza viral protein NS1, ISG56, and β-Actin. (B) Viral titers in cell culture supernatants from (A) were determined by plaque assay. Error bars represent SEM from two independent samples. (C) A549 cells were pre-treated with increasing concentrations of 5′pppRNA (0.1 ng/ml to 10 ng/ml) for 24 h, prior to 0.2MOI influenza challenge. WCEs were examined for influenza NS1, ISG56, β-Actin by immunoblot. (D) Viral titers in cell culture supernatants from (C) were determined by plaque assay. Error bars represent SEM from two independent samples. (E) A549 cells were transfected with a control siRNA, RIG-I siRNA or IFNα/βR siRNA and then treated with 5′pppRNA (10 ng/ml), followed by infection with Influenza (0.2MOI). WCEs were prepared 24 h later and subjected to SDS PAGE and immunoblot to examine the expression of influenza NS1, RIG-I, IFIT1, and β-Actin. (F) A549 cells were transfected with a control siRNA or IFNα/βR siRNA and then treated with 5′pppRNA (10 ng/ml) or IFNα-2b (100 IU/mL) for 24 h. Expression of IFIT1, RIG-I and β-Actin was evaluated by western blotting.
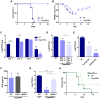
Figure 8. 5′pppRNA activates innate immunity and protects mice from lethal influenza infection in vivo.
C57Bl/6 mice were injected intravenously with 25 ug of 5′pppRNA in complex with in vivo-jetPEI. (A–B–C) Mice were treated with 5′pppRNA on the day prior to influenza infection (500 PFU; Day −1) and the day of infection (Day 0). Percent survival (A) and percent weight loss (B) were monitored. (C) Lung viral titers were measured by plaque assay at the indicated day post-infection. Error bars represent SEM from six different animals. ND: not detected. (D) Repetitive administration of 5′pppRNA on the indicated days further decreases lung viral titers following infection with 500 PFU of influenza, as determined by plaque assay on Day 3 post-infection. Error bars represent SEM from five different animals. (E) Mice were infected with 50 PFU of influenza on Day 0. 5′pppRNA was administered prophylactically (Day −1, Day 0) or therapeutically (Day 1, Day 2) and lung viral titers were determined on Day 3. Error bars represent SEM from five different animals. (F) WT, TLR3−/−, MAVS−/− mice were treated with 5′pppRNA and serum IFNβ was quantified by ELISA at 6 h. Error bars represent SEM from three different animals. (G) WT and MAVS−/− mice were treated with 5′pppRNA and infected with influenza (500 PFU). Lungs were collected and homogenized on Day 1 and lung viral titers were determined by plaque assay. Error bars represent SEM from four different animals. (H) IFNα/βR−/− mice were either non-treated or treated with 5′pppRNA and infected with influenza (100 PFU). Survival was monitored for 18 days. Statistical analysis was performed by Student's t test (*, p≤0.05; **, p≤0.01; ***, p≤0.001; ns, not statistically significant).
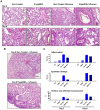
Figure 9. 5′pppRNA treatment controls influenza-mediated pneumonia.
Mice were treated with 5′pppRNA in complex in vivo-jetPEI on the day prior to influenza infection (Day −1) and the day of infection (Day 0). Lungs were collected on Day 3 and Day 8 post-infection and stained with hematoxylin and eosin (H&E). For each group, a representative picture showing (A) inflammation and tissue damage and (B) the extent of pneumonia is presented. (C) Inflammation, tissue damage and surface area affected by pneumonia were scored by a veterinary pathologist. Grade 1 = minimal; Grade 2 = modest, rare; Grade 3 = moderate, frequent; Grade 4 = severe, extensive.
Similar articles
- Sequence-Specific Modifications Enhance the Broad-Spectrum Antiviral Response Activated by RIG-I Agonists.
Chiang C, Beljanski V, Yin K, Olagnier D, Ben Yebdri F, Steel C, Goulet ML, DeFilippis VR, Streblow DN, Haddad EK, Trautmann L, Ross T, Lin R, Hiscott J. Chiang C, et al. J Virol. 2015 Aug;89(15):8011-25. doi: 10.1128/JVI.00845-15. Epub 2015 May 27. J Virol. 2015. PMID: 26018150 Free PMC article. - Inhibition of dengue and chikungunya virus infections by RIG-I-mediated type I interferon-independent stimulation of the innate antiviral response.
Olagnier D, Scholte FE, Chiang C, Albulescu IC, Nichols C, He Z, Lin R, Snijder EJ, van Hemert MJ, Hiscott J. Olagnier D, et al. J Virol. 2014 Apr;88(8):4180-94. doi: 10.1128/JVI.03114-13. Epub 2014 Jan 29. J Virol. 2014. PMID: 24478443 Free PMC article. - Coxsackievirus cloverleaf RNA containing a 5' triphosphate triggers an antiviral response via RIG-I activation.
Feng Q, Langereis MA, Olagnier D, Chiang C, van de Winkel R, van Essen P, Zoll J, Hiscott J, van Kuppeveld FJ. Feng Q, et al. PLoS One. 2014 Apr 23;9(4):e95927. doi: 10.1371/journal.pone.0095927. eCollection 2014. PLoS One. 2014. PMID: 24759703 Free PMC article. - Standing on three legs: antiviral activities of RIG-I against influenza viruses.
Weber-Gerlach M, Weber F. Weber-Gerlach M, et al. Curr Opin Immunol. 2016 Oct;42:71-75. doi: 10.1016/j.coi.2016.05.016. Epub 2016 Jun 16. Curr Opin Immunol. 2016. PMID: 27318973 Review. - Defense genes missing from the flight division.
Magor KE, Miranzo Navarro D, Barber MR, Petkau K, Fleming-Canepa X, Blyth GA, Blaine AH. Magor KE, et al. Dev Comp Immunol. 2013 Nov;41(3):377-88. doi: 10.1016/j.dci.2013.04.010. Epub 2013 Apr 24. Dev Comp Immunol. 2013. PMID: 23624185 Free PMC article. Review.
Cited by
- A Short 5'triphosphate RNA nCoV-L Induces a Broad-Spectrum Antiviral Response by Activating RIG-I.
Song Z, Wang Q, Bian L, An C, Cui B, Mao Q, Wu X, He Q, Bai Y, Liu J, Song L, Liu D, Zhang J, Gao F, Li X, Liang Z. Song Z, et al. Viruses. 2022 Nov 4;14(11):2451. doi: 10.3390/v14112451. Viruses. 2022. PMID: 36366549 Free PMC article. - RIG-I ATPase activity and discrimination of self-RNA versus non-self-RNA.
Anchisi S, Guerra J, Garcin D. Anchisi S, et al. mBio. 2015 Mar 3;6(2):e02349. doi: 10.1128/mBio.02349-14. mBio. 2015. PMID: 25736886 Free PMC article. - RIG-I in RNA virus recognition.
Kell AM, Gale M Jr. Kell AM, et al. Virology. 2015 May;479-480:110-21. doi: 10.1016/j.virol.2015.02.017. Epub 2015 Mar 5. Virology. 2015. PMID: 25749629 Free PMC article. Review. - An optimized retinoic acid-inducible gene I agonist M8 induces immunogenic cell death markers in human cancer cells and dendritic cell activation.
Castiello L, Zevini A, Vulpis E, Muscolini M, Ferrari M, Palermo E, Peruzzi G, Krapp C, Jakobsen M, Olagnier D, Zingoni A, Santoni A, Hiscott J. Castiello L, et al. Cancer Immunol Immunother. 2019 Sep;68(9):1479-1492. doi: 10.1007/s00262-019-02380-2. Epub 2019 Aug 28. Cancer Immunol Immunother. 2019. PMID: 31463653 Free PMC article. - Rigging Innate Immunity against the Flu.
Krieg AM. Krieg AM. Mol Ther. 2017 Sep 6;25(9):1993-1994. doi: 10.1016/j.ymthe.2017.08.008. Epub 2017 Aug 23. Mol Ther. 2017. PMID: 28844477 Free PMC article. No abstract available.
References
- Yoneyama M, Fujita T (2010) Recognition of viral nucleic acids in innate immunity. Rev Med Virol 20: 4–22. - PubMed
- Brennan K, Bowie AG (2010) Activation of host pattern recognition receptors by viruses. Curr Opin Microbiol 13: 503–507. - PubMed
- Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140: 805–820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous