ROS production and NF-κB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation - PubMed (original) (raw)
. 2013 Jun 6;12(6):761-73.
doi: 10.1016/j.stem.2013.04.006. Epub 2013 May 9.
Patrizia Cammareri, Ewan J McGhee, Rachel A Ridgway, David J Huels, Julia B Cordero, Sarah Schwitalla, Gabriela Kalna, Erinn-Lee Ogg, Dimitris Athineos, Paul Timpson, Marcos Vidal, Graeme I Murray, Florian R Greten, Kurt I Anderson, Owen J Sansom
Affiliations
- PMID: 23665120
- PMCID: PMC3690525
- DOI: 10.1016/j.stem.2013.04.006
ROS production and NF-κB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation
Kevin B Myant et al. Cell Stem Cell. 2013.
Abstract
The Adenomatous Polyposis Coli (APC) gene is mutated in the majority of colorectal cancers (CRCs). Loss of APC leads to constitutively active WNT signaling, hyperproliferation, and tumorigenesis. Identification of pathways that facilitate tumorigenesis after APC loss is important for therapeutic development. Here, we show that RAC1 is a critical mediator of tumorigenesis after APC loss. We find that RAC1 is required for expansion of the LGR5 intestinal stem cell (ISC) signature, progenitor hyperproliferation, and transformation. Mechanistically, RAC1-driven ROS and NF-κB signaling mediate these processes. Together, these data highlight that ROS production and NF-κB activation triggered by RAC1 are critical events in CRC initiation.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Graphical abstract
Figure 1
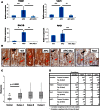
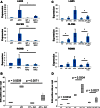
RAC1 Activity Is Increased after Apc Loss (A) qRT-PCR of Tiam1, Vav3, Rac1b, and Rac1 (error bars represent SD; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; t test, n = 3). (B) IHC for RAC-GTP in WT, APC, APC Myc, and Rac1 intestines. Red arrows indicate epithelial expression, and black arrows show immune cells in the villus compartment with very high positivity. (C) Histoscores of human CRC TMA cores stained for RAC-GTP (Mann Whitney). (D) Table comparing levels of active RAC, TIAM1, VAV3, and MYC in human CRC samples. All experiments are 4 days after induction. Scale bars represent 100 μm. See also Figure S1.
Figure 2
RAC1 Is Required for Hyperproliferation and LGR5 ISC Expansion after Apc Loss (A) Quantification of BrdU IHC in WT, Rac1, APC, and APC Rac1 intestines (Mann Whitney, n = 4). (B) β-catenin IHC on WT, Rac1, APC, and APC Rac1 intestines. Nuclear β-catenin is found throughout the crypt in APC and APC Rac1 tissue (black arrows). Histoscoring identified a significant increase in nuclear positivity after Apc loss (p = 0.04, Mann Whitney, n = 3) that was unchanged in APC Rac1. (C) qRT-PCR of three ISC markers (error bars represent SD; t test, n = 3, ∗p < 0.05, ∗∗p < 0.01). (D) Quantification of LGR5-GFP+ cell numbers. (E) Cumulative frequency scoring of LGR5-GFP cell position (error bars represent SEM). All experiments 4 days after induction. Scale bars represent 100 μm. See also Figure S2 and Tables S1 and S2.
Figure 3
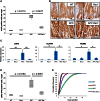
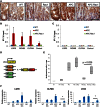
RAC1 Is Required for ROS Production in the Intestine (A) Dihydroethidium (DHE) staining of frozen sections from WT, Rac1, APC, and APC Rac1 intestines; DAPI counterstain. (B) Quanitification of DHE staining (error bars represent SD; t test, n = 3, ∗p < 0.05, ∗∗p < 0.01). (C) Representative fluorescence-activated cell sorting (FACS) plot of CellROX Deep Red-stained epithelial cells from an Lgr5 _GFP-CREER_-expressing mouse. (D) Quantification of CellROX Deep Red staining (error bars represent SEM; t test, n = 3, ∗∗∗p < 0.001). (E) Expression of Rac signaling components and control ISC markers in LGR5-GFP+ cells relative to LGR5-GFP− cells (error bars represent SEM; t test, n = 3, ∗p < 0.05, ∗∗p < 0.01). See also Figure S3.
Figure 4
ROS Is Required for Expansion of the ISC Signature (A) qRT-PCR of three ISC markers (error bars represent SD; t test, n = 3, ∗p < 0.05, ∗∗p < 0.01). (B) Quantification of BrdU staining of intestinal crypts from WT, APC, WT + NAC, and APC + NAC mice (Mann Whitney, n = 4). (C) qRT-PCR of three ISC markers, downregulated in APC Rac1-deficient intestines (AR) and upregulated upon treatment with paraquat (AR + PQ) (error bars represent SD; t test, n = 3, ∗p < 0.05, ∗∗∗p < 0.001). APC + PQ has been removed for clarity and is included in Figure S4C. (D) Quantification of BrdU positivity (Mann Whitney, n = 6). All experiments are 4 days after induction. Scale bars represent 100 μm. See also Figure S4.
Figure 5
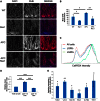
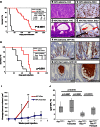
NF-κB Signaling after Apc Loss Requires RAC1 (A) P65 IHC shows decreased expression in APC Rac1 crypts. (B) ChIP of P65 to the Lgr5, Olfm4, and Rgmb promoters and a control region (error bars represent SD; Mann Whitney, n = 3, p = 0.04). (C) Control ChIP showing no binding to ISC promoters of a nonspecific IgG (error bars represent SD). (D) Crossing strategy to generate Vil APC Rac1 IKK mice. (E) Quantification of BrdU positivity (Mann Whitney, n = 4). (F) qRT-PCR of three ISC markers showing increased expression in APC Rac1 mice after NF-κB activation (error bars represent SEM; t test, n = 3, ∗p < 0.05). All experiments were performed 4 days after induction. Scale bars represent 100 μm. See also Figure S5.
Figure 6
RAC1 Is Required for Tumorigenesis Downstream of Apc Loss (A) Compared to Lgr5 APC, Lgr5 APC Rac1 mice were strongly protected against tumorigenesis (Kaplan Meier, p < 0.0001, n = 15). (B and C) Lgr5 APC mice developed adenomas (B, H&E) that were highly proliferative (C, BrdU). (D and E) Lgr5 APC Rac1 mice predominantly developed small intestinal lesions or cysts (D, H&E) that were poorly proliferative (E, BrdU IHC, red arrows). BrdU incorporation was higher in neighboring normal intestine (black arrows). (F and G) GFP IHC showing high levels of Lgr5-GFP staining in Lgr5 APC adenomas (F) compared to Lgr5 APC Rac1 lesions (G). (H) NAC treatment protected Lgr5 APC mice from intestinal tumorigenesis (Kaplan Meier, p = 0.001, n = 7). (I) Tumor volume scores of allografts derived from 100 APC Kras and APC Kras Rac1-purified crypts (error bars represent SD; t test, n = 3, ∗p < 0.05, ∗∗p < 0.01). (J) Quantification of FACS analysis demonstrating a significant reduction in LGR5-GFP positivity in tumors deficient in RAC1 (Mann-Whitney, n ≥ 5). Scale bars represent 200 μm. See also Figure S6.
Figure 7
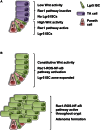
Model of RAC1-Mediated Control of Intestinal Tumor Initiation (A) Normal small intestinal crypt with different cell populations outlined. LGR5 ISCs have high Wnt signaling leading to high RAC1-ROS-NF-κB pathway activation. (B) Upon loss of Apc, RAC1-ROS-NF-κB pathway activation is increased throughout the crypt, permitting the expansion of the LGR5 ISC zone and progenitor cell hyperproliferation. The activation of the RAC1-ROS-NF-κB pathway throughout the crypt is critical for intestinal adenoma formation.
Comment in
- The other face of ROS: a driver of stem cell expansion in colorectal cancer.
Pelicci PG, Dalton P, Giorgio M. Pelicci PG, et al. Cell Stem Cell. 2013 Jun 6;12(6):635-6. doi: 10.1016/j.stem.2013.05.023. Cell Stem Cell. 2013. PMID: 23746969
Similar articles
- The other face of ROS: a driver of stem cell expansion in colorectal cancer.
Pelicci PG, Dalton P, Giorgio M. Pelicci PG, et al. Cell Stem Cell. 2013 Jun 6;12(6):635-6. doi: 10.1016/j.stem.2013.05.023. Cell Stem Cell. 2013. PMID: 23746969 - Opposing effects of TIGAR- and RAC1-derived ROS on Wnt-driven proliferation in the mouse intestine.
Cheung EC, Lee P, Ceteci F, Nixon C, Blyth K, Sansom OJ, Vousden KH. Cheung EC, et al. Genes Dev. 2016 Jan 1;30(1):52-63. doi: 10.1101/gad.271130.115. Epub 2015 Dec 17. Genes Dev. 2016. PMID: 26679840 Free PMC article. - Rac1 drives intestinal stem cell proliferation and regeneration.
Myant KB, Scopelliti A, Haque S, Vidal M, Sansom OJ, Cordero JB. Myant KB, et al. Cell Cycle. 2013 Sep 15;12(18):2973-7. doi: 10.4161/cc.26031. Epub 2013 Aug 12. Cell Cycle. 2013. PMID: 23974108 Free PMC article. - The adenomatous polyposis coli tumor suppressor and Wnt signaling in the regulation of apoptosis.
Benchabane H, Ahmed Y. Benchabane H, et al. Adv Exp Med Biol. 2009;656:75-84. doi: 10.1007/978-1-4419-1145-2_7. Adv Exp Med Biol. 2009. PMID: 19928354 Free PMC article. Review. - Interaction of the Wnt/β-catenin and RAS-ERK pathways involving co-stabilization of both β-catenin and RAS plays important roles in the colorectal tumorigenesis.
Lee SK, Hwang JH, Choi KY. Lee SK, et al. Adv Biol Regul. 2018 May;68:46-54. doi: 10.1016/j.jbior.2018.01.001. Epub 2018 Jan 10. Adv Biol Regul. 2018. PMID: 29449169 Review.
Cited by
- Intestinal stem cell niche: An upcoming area of immense importance in gastrointestinal disorders.
Mehra L, Bhowmik S, Makharia GK, Das P. Mehra L, et al. Indian J Gastroenterol. 2024 Nov 8. doi: 10.1007/s12664-024-01699-8. Online ahead of print. Indian J Gastroenterol. 2024. PMID: 39514159 Review. - Improving data interpretability with new differential sample variance gene set tests.
Rahmatallah Y, Glazko G. Rahmatallah Y, et al. Res Sq [Preprint]. 2024 Sep 9:rs.3.rs-4888767. doi: 10.21203/rs.3.rs-4888767/v1. Res Sq. 2024. PMID: 39315246 Free PMC article. Preprint. - NRF2 in age-related musculoskeletal diseases: Role and treatment prospects.
Zhang X, Li H, Chen L, Wu Y, Li Y. Zhang X, et al. Genes Dis. 2023 Nov 27;11(6):101180. doi: 10.1016/j.gendis.2023.101180. eCollection 2024 Nov. Genes Dis. 2023. PMID: 39281838 Free PMC article. Review. - Loss of DOCK2 potentiates Inflammatory Bowel Disease-associated colorectal cancer via immune dysfunction and IFNγ induction of IDO1 expression.
Churchhouse AMD, Billard CV, Suzuki T, Pohl SÖG, Doleschall NJ, Donnelly K, Nixon C, Arends MJ, Din S, Kirkwood K, Marques Junior J, Von Kriegsheim A, Coffelt SB, Myant KB. Churchhouse AMD, et al. Oncogene. 2024 Oct;43(42):3094-3107. doi: 10.1038/s41388-024-03135-9. Epub 2024 Sep 7. Oncogene. 2024. PMID: 39242821 Free PMC article. - Oxygen vacancy-engineered cerium oxide mediated by copper-platinum exhibit enhanced SOD/CAT-mimicking activities to regulate the microenvironment for osteoarthritis therapy.
Yang J, Xiao S, Deng J, Li Y, Hu H, Wang J, Lu C, Li G, Zheng L, Wei Q, Zhong J. Yang J, et al. J Nanobiotechnology. 2024 Aug 18;22(1):491. doi: 10.1186/s12951-024-02678-z. J Nanobiotechnology. 2024. PMID: 39155382 Free PMC article.
References
- Athineos D., Sansom O.J. Myc heterozygosity attenuates the phenotypes of APC deficiency in the small intestine. Oncogene. 2010;29:2585–2590. - PubMed
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
- Barker N., Ridgway R.A., van Es J.H., van de Wetering M., Begthel H., van den Born M., Danenberg E., Clarke A.R., Sansom O.J., Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. - PubMed
- Bromberg Y., Shani E., Joseph G., Gorzalczany Y., Sperling O., Pick E. The GDP-bound form of the small G protein Rac1 p21 is a potent activator of the superoxide-forming NADPH oxidase of macrophages. J. Biol. Chem. 1994;269:7055–7058. - PubMed
- Buczacki S.J., Zecchini H.I., Nicholson A.M., Russell R., Vermeulen L., Kemp R., Winton D.J. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 12481/CRUK_/Cancer Research UK/United Kingdom
- 15565/CRUK_/Cancer Research UK/United Kingdom
- G1000078/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials