Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity - PubMed (original) (raw)
. 2013 May 28;110(22):9066-71.
doi: 10.1073/pnas.1219451110. Epub 2013 May 13.
Clara Belzer, Lucie Geurts, Janneke P Ouwerkerk, Céline Druart, Laure B Bindels, Yves Guiot, Muriel Derrien, Giulio G Muccioli, Nathalie M Delzenne, Willem M de Vos, Patrice D Cani
Affiliations
- PMID: 23671105
- PMCID: PMC3670398
- DOI: 10.1073/pnas.1219451110
Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity
Amandine Everard et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Obesity and type 2 diabetes are characterized by altered gut microbiota, inflammation, and gut barrier disruption. Microbial composition and the mechanisms of interaction with the host that affect gut barrier function during obesity and type 2 diabetes have not been elucidated. We recently isolated Akkermansia muciniphila, which is a mucin-degrading bacterium that resides in the mucus layer. The presence of this bacterium inversely correlates with body weight in rodents and humans. However, the precise physiological roles played by this bacterium during obesity and metabolic disorders are unknown. This study demonstrated that the abundance of A. muciniphila decreased in obese and type 2 diabetic mice. We also observed that prebiotic feeding normalized A. muciniphila abundance, which correlated with an improved metabolic profile. In addition, we demonstrated that A. muciniphila treatment reversed high-fat diet-induced metabolic disorders, including fat-mass gain, metabolic endotoxemia, adipose tissue inflammation, and insulin resistance. A. muciniphila administration increased the intestinal levels of endocannabinoids that control inflammation, the gut barrier, and gut peptide secretion. Finally, we demonstrated that all these effects required viable A. muciniphila because treatment with heat-killed cells did not improve the metabolic profile or the mucus layer thickness. In summary, this study provides substantial insight into the intricate mechanisms of bacterial (i.e., A. muciniphila) regulation of the cross-talk between the host and gut microbiota. These results also provide a rationale for the development of a treatment that uses this human mucus colonizer for the prevention or treatment of obesity and its associated metabolic disorders.
Keywords: LPS; Lactobacillus plantarum; RegIIIγ; antimicrobial peptides; gut permeability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
A. muciniphila abundance is decreased in obese and diabetic mice, and prebiotic treatment restored A. muciniphila to basal levels and reversed metabolic endotoxemia and related disorders. (A) A. muciniphila abundance (log10 of bacteria per g of cecal content) measured in the cecal content of leptin-deficient (ob-ob) obese mice and their lean littermates (lean) (n = 5). (B) A. muciniphila abundance (log10 of bacteria per g of cecal content) measured in the cecal content of control diet-fed mice (CT) or CT diet-fed mice treated with prebiotics (CT-Pre) added to their drinking water and HF diet-fed mice (HF) or HF diet-fed mice treated with prebiotics (HF-Pre) added to their drinking water for 8 wk (n = 10). (C) A. muciniphila abundance (log10 of bacteria per g of cecal content) measured in the cecal content of obese mice fed a control diet (ob-CT) or treated with prebiotics (ob-Pre) for 5 wk (n = 10). (D) Portal vein serum LPS levels (n = 7–9). (E) mRNA expression of the adipose tissue macrophage infiltration marker CD11c (n = 10). (F) Total fat mass gain measured by time-domain NMR (n = 10). (G) Pearson’s correlation between log values of portal vein LPS levels and A. muciniphila abundance (log10 of bacteria per g of cecal content); (Inset) Pearson’s correlation coefficient (r) and the corresponding P value. Data are shown as means ± SEM; *P < 0.05 by two-tailed Student t test, data with different superscript letters are significantly different (P < 0.05) according to post hoc ANOVA one-way statistical analysis.
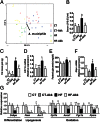
Fig. 2.
A. muciniphila counteracted metabolic endotoxemia, diet-induced obesity, adipose tissue macrophage infiltration, improved glucose homeostasis, and adipose tissue metabolism in diet-induced obese mice without modifying gut microbiota composition. (A) Principal component analysis using the MITChip phylogenetic fingerprints of the gut microbiota from the cecal contents of control mice treated with a daily oral gavage containing sterile anaerobic PBS for 4 wk and fed a control (CT) or HF diet (HF) (CT in red and HF in green) and in mice treated with a daily oral gavage containing A. muciniphila (2.108 bacterial cells suspended in 200 µL of sterile anaerobic PBS) and fed a control (CT-Akk) or HF diet (HF-Akk) (CT-Akk in blue and HF-Akk in yellow) (n = 10). (B) Portal vein serum LPS levels (n = 6–10). (C) Total fat mass gain measured by time-domain NMR (n = 10). (D) mRNA expression of the adipose tissue macrophage infiltration marker CD11c (n = 10). (E) Fasting glycemia (n = 10). (F) Liver G6pc mRNA (n = 10). (G) mRNA expression of markers of adipocyte differentiation (Cebpa), lipogenesis (Acc1; Fasn), and lipid oxidation (Cpt1; Acox1; Pgc1a; and Ppara) was measured in visceral fat depots (mesenteric fat) (n = 10). Data are shown as means ± SEM. Data with different superscript letters are significantly different (P < 0.05) according to post hoc ANOVA one-way statistical analysis.
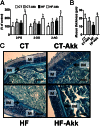
Fig. 3.
A. muciniphila colonization restored gut barrier function and increased intestinal endocannabinoids in diet-induced obese mice. (A) Ileum 2-PG, 2-OG, and 2-AG (expressed as percentage of the control) (n = 10). (B) Thickness of the mucus layer measured by histological analyses After alcian blue staining (n = 7–8). (C) Representative alcian blue images that were used for mucus layer thickness measurements. M, mucosa; IM, inner mucus layer. (Scale bars, 40 µm.) Data are shown as means ± SEM. Data with different superscript letters are significantly different (P < 0.05) according to post hoc ANOVA one-way statistical analysis.
Fig. 4.
Heat-killed A. muciniphila did not counteract metabolic endotoxemia, diet-induced obesity, oral glucose intolerance, and did not improve adipose tissue metabolism and gut barrier function in diet-induced obese mice. Control mice were fed a control (CT) or HF diet (HF) and treated with a daily oral gavage containing sterile anaerobic PBS and glycerol for 4 wk daily. Treated mice received an oral gavage of alive A. muciniphila (HF-Akk) or killed A. muciniphila (HF-K-Akk) (2.108 bacterial cells suspended in 200 µL of sterile anaerobic PBS) and fed an HF diet (n = 8). (A) Portal vein serum LPS levels (n = 6–7). (B) Total fat mass gain measured by time-domain NMR (n = 7–8). (C) Plasma glucose profile after 2 g/kg glucose oral challenge in freely moving mice. (Inset) Mean area under the curve (AUC) measured between 0 and 120 min after glucose load (n = 7–8). (D) mRNA expression of markers of adipocyte differentiation (Cebpa), lipogenesis (Acc1; Fasn), and lipid oxidation (Cpt1; Acox1; Pgc1a; and Ppara) was measured in visceral fat depots (mesenteric fat) (n = 8). (E) Thickness of the mucus layer measured by histological analyses after alcian blue staining (CT n = 4, HF n = 6, HF-Akk and HF-K-Akk n = 5). (F) Representative alcian blue images that were used for mucus layer thickness measurements. M, mucosa; IM, inner mucus layer. (Scale bars, 40 µm.) Data are shown as means ± SEM. Data with different superscript letters are significantly different (P < 0.05) according to post hoc ANOVA one-way statistical analysis.
Comment in
- Microbiome: A mucus colonizer manages host metabolism.
Hofer U. Hofer U. Nat Rev Microbiol. 2013 Jul;11(7):430-1. doi: 10.1038/nrmicro3051. Epub 2013 May 28. Nat Rev Microbiol. 2013. PMID: 23712351 Free PMC article. No abstract available.
Similar articles
- Heat-Inactivated Akkermansia muciniphila Improves Gut Permeability but Does Not Prevent Development of Non-Alcoholic Steatohepatitis in Diet-Induced Obese Ldlr-/-.Leiden Mice.
Morrison MC, Gart E, Duyvenvoorde WV, Snabel J, Nielsen MJ, Leeming DJ, Menke A, Kleemann R. Morrison MC, et al. Int J Mol Sci. 2022 Feb 19;23(4):2325. doi: 10.3390/ijms23042325. Int J Mol Sci. 2022. PMID: 35216439 Free PMC article. - Akkermansia muciniphila: key player in metabolic and gastrointestinal disorders.
Macchione IG, Lopetuso LR, Ianiro G, Napoli M, Gibiino G, Rizzatti G, Petito V, Gasbarrini A, Scaldaferri F. Macchione IG, et al. Eur Rev Med Pharmacol Sci. 2019 Sep;23(18):8075-8083. doi: 10.26355/eurrev_201909_19024. Eur Rev Med Pharmacol Sci. 2019. PMID: 31599433 Review. - Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice.
Schneeberger M, Everard A, Gómez-Valadés AG, Matamoros S, Ramírez S, Delzenne NM, Gomis R, Claret M, Cani PD. Schneeberger M, et al. Sci Rep. 2015 Nov 13;5:16643. doi: 10.1038/srep16643. Sci Rep. 2015. PMID: 26563823 Free PMC article. - Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions.
Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, Jeon J, Kim MS, Jee YK, Gho YS, Park HS, Kim YK, Ryu SH. Chelakkot C, et al. Exp Mol Med. 2018 Feb 23;50(2):e450. doi: 10.1038/emm.2017.282. Exp Mol Med. 2018. PMID: 29472701 Free PMC article. - Akkermansia muciniphila as a novel powerful bacterial player in the treatment of metabolic disorders.
Kobyliak N, Falalyeyeva T, Kyriachenko Y, Tseyslyer Y, Kovalchuk O, Hadiliia O, Eslami M, Yousefi B, Abenavoli L, Fagoonee S, Pellicano R. Kobyliak N, et al. Minerva Endocrinol (Torino). 2022 Jun;47(2):242-252. doi: 10.23736/S2724-6507.22.03752-6. Epub 2022 Feb 1. Minerva Endocrinol (Torino). 2022. PMID: 35103461 Review.
Cited by
- Fetal programming by the parental microbiome of offspring behavior, and DNA methylation and gene expression within the hippocampus.
Gustafson KL, Busi SB, McAdams ZL, McCorkle RE, Khodakivskyi P, Bivens NJ, Davis DJ, Raju M, Coghill LM, Goun EA, Amos-Landgraf J, Franklin CL, Wilmes P, Cortese R, Ericsson AC. Gustafson KL, et al. bioRxiv [Preprint]. 2024 Oct 22:2024.04.12.589237. doi: 10.1101/2024.04.12.589237. bioRxiv. 2024. PMID: 39484583 Free PMC article. Preprint. - Gut Microbiota Composition in Undernourished Children Associated with Diet and Sociodemographic Factors: A Case-Control Study in Indonesia.
Gatya M, Fibri DLN, Utami T, Suroto DA, Rahayu ES. Gatya M, et al. Microorganisms. 2022 Aug 30;10(9):1748. doi: 10.3390/microorganisms10091748. Microorganisms. 2022. PMID: 36144350 Free PMC article. - Akkermansia muciniphila Suppresses High-Fat Diet-Induced Obesity and Related Metabolic Disorders in Beagles.
Lin XQ, Chen W, Ma K, Liu ZZ, Gao Y, Zhang JG, Wang T, Yang YJ. Lin XQ, et al. Molecules. 2022 Sep 17;27(18):6074. doi: 10.3390/molecules27186074. Molecules. 2022. PMID: 36144806 Free PMC article. - Fermented Soybean Meal (FSBM) in African Catfish (Clarias gariepinus) Diets: Effects on Growth Performance, Fish Gut Microbiota Analysis, Blood Haematology, and Liver Morphology.
Zakaria MK, Kari ZA, Van Doan H, Kabir MA, Che Harun H, Mohamad Sukri SA, Goh KW, Wee W, Khoo MI, Wei LS. Zakaria MK, et al. Life (Basel). 2022 Nov 11;12(11):1851. doi: 10.3390/life12111851. Life (Basel). 2022. PMID: 36430986 Free PMC article. - Metformin: update on mechanisms of action on liver diseases.
Ruan G, Wu F, Shi D, Sun H, Wang F, Xu C. Ruan G, et al. Front Nutr. 2023 Dec 14;10:1327814. doi: 10.3389/fnut.2023.1327814. eCollection 2023. Front Nutr. 2023. PMID: 38192642 Free PMC article. Review.
References
- Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. - PubMed
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18(3):363–374. - PubMed
- Cani PD, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical