Reducing haemorrhagic transformation after thrombolysis for stroke: a strategy utilising minocycline - PubMed (original) (raw)
Reducing haemorrhagic transformation after thrombolysis for stroke: a strategy utilising minocycline
David J Blacker et al. Stroke Res Treat. 2013.
Abstract
Haemorrhagic transformation (HT) of recently ischaemic brain is a feared complication of thrombolytic therapy that may be caused or compounded by ischaemia-induced activation of matrix metalloproteinases (MMPs). The tetracycline antibiotic minocycline inhibits matrix MMPs and reduces macroscopic HT in rodents with stroke treated with tissue plasminogen activator (tPA). The West Australian Intravenous Minocycline and TPA Stroke Study (WAIMATSS) aims to determine the safety and efficacy of adding minocycline to tPA in acute ischaemic stroke. The WAIMATSS is a multicentre, prospective, and randomised pilot study of intravenous minocycline, 200 mg 12 hourly for 5 doses, compared with standard care, in patients with ischaemic stroke treated with intravenous tPA. The primary endpoint is HT diagnosed by brain CT and MRI. Secondary endpoints include clinical outcome measures. Some illustrative cases from the early recruitment phase of this study will be presented, and future perspectives will be discussed.
Figures
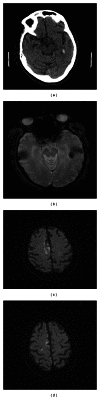
Figure 1
Subject presented with acute left hemiparesis, leg more affected than arm. Routine day one CT (a) and day 5 MRI (b) demonstrate a small haemorrhage in the left temporal lobe, away from the main area of infarction, shown on diffusion weighted MRI (c) and (d), mainly in the territory of the right anterior cerebral artery. No clinical deterioration was detected.
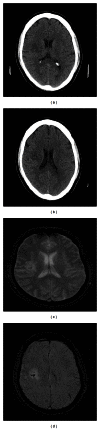
Figure 2
Subject presented with mild left hemiparesis. The day one CT (a) and (b) shows a small infarct in the right subcortical white matter, with a suggestion of increased signal within the central portion. The gradient echo (c) and susceptibility weighted image (d) MRI sequences performed on day seven suggest a small amount of blood within the infarct. No clinical deterioration was detected.
Similar articles
- Regulatory T cells ameliorate tissue plasminogen activator-induced brain haemorrhage after stroke.
Mao L, Li P, Zhu W, Cai W, Liu Z, Wang Y, Luo W, Stetler RA, Leak RK, Yu W, Gao Y, Chen J, Chen G, Hu X. Mao L, et al. Brain. 2017 Jul 1;140(7):1914-1931. doi: 10.1093/brain/awx111. Brain. 2017. PMID: 28535201 Free PMC article. - Outcome of stroke patients receiving different doses of recombinant tissue plasminogen activator.
Ong CT, Wong YS, Wu CS, Su YH. Ong CT, et al. Drug Des Devel Ther. 2017 May 18;11:1559-1566. doi: 10.2147/DDDT.S133759. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28572721 Free PMC article. - Absolute blood eosinophil count could be a potential biomarker for predicting haemorrhagic transformation after intravenous thrombolysis for acute ischaemic stroke.
Jucevičiūtė N, Mikužis P, Balnytė R. Jucevičiūtė N, et al. BMC Neurol. 2019 Jun 13;19(1):127. doi: 10.1186/s12883-019-1359-6. BMC Neurol. 2019. PMID: 31195995 Free PMC article. - Thrombolysis for acute ischaemic stroke.
Wardlaw JM, Zoppo G, Yamaguchi T, Berge E. Wardlaw JM, et al. Cochrane Database Syst Rev. 2003;(3):CD000213. doi: 10.1002/14651858.CD000213. Cochrane Database Syst Rev. 2003. PMID: 12917889 Updated. Review. - Low-dose intravenous tissue plasminogen activator for acute ischaemic stroke: an alternative or a new standard?
Dong Y, Cao W, Cheng X, Fang K, Wu F, Yang L, Xie Y, Dong Q. Dong Y, et al. Stroke Vasc Neurol. 2016 Oct 25;1(3):115-121. doi: 10.1136/svn-2016-000033. eCollection 2016 Sep. Stroke Vasc Neurol. 2016. PMID: 28959472 Free PMC article. Review.
Cited by
- Resilience to Injury: A New Approach to Neuroprotection?
Singhal NS, Sun CH, Lee EM, Ma DK. Singhal NS, et al. Neurotherapeutics. 2020 Apr;17(2):457-474. doi: 10.1007/s13311-020-00832-7. Neurotherapeutics. 2020. PMID: 31997268 Free PMC article. Review. - Combination therapy for ischemic stroke: Novel approaches to lengthen therapeutic window of tissue plasminogen activator.
Knecht T, Borlongan C, Dela Peña I. Knecht T, et al. Brain Circ. 2018 Jul-Sep;4(3):99-108. doi: 10.4103/bc.bc_21_18. Epub 2018 Oct 9. Brain Circ. 2018. PMID: 30450415 Free PMC article. Review. - Haemorrhagic transformation following ischaemic stroke: A retrospective study.
Pande SD, Win MM, Khine AA, Zaw EM, Manoharraj N, Lolong L, Tin AS. Pande SD, et al. Sci Rep. 2020 Mar 24;10(1):5319. doi: 10.1038/s41598-020-62230-5. Sci Rep. 2020. PMID: 32210323 Free PMC article. - Clinical trials in acute ischemic stroke.
Kikuchi K, Tanaka E, Murai Y, Tancharoen S. Kikuchi K, et al. CNS Drugs. 2014 Oct;28(10):929-38. doi: 10.1007/s40263-014-0199-6. CNS Drugs. 2014. PMID: 25160686 Review. - Strategies to Extend Thrombolytic Time Window for Ischemic Stroke Treatment: An Unmet Clinical Need.
Peña ID, Borlongan C, Shen G, Davis W. Peña ID, et al. J Stroke. 2017 Jan;19(1):50-60. doi: 10.5853/jos.2016.01515. Epub 2017 Jan 31. J Stroke. 2017. PMID: 28178410 Free PMC article. Review.
References
- Derex L, Nighoghossian N. Intracerebral haemorrhage after thrombolysis for acute ischaemic stroke: an update. Journal of Neurology, Neurosurgery and Psychiatry. 2008;79(10):1093–1099. - PubMed
- Wahlgren N, Ahmed N, Dávalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. The Lancet. 2007;369(9558):275–282. - PubMed
- Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II) The Lancet. 1998;352(9136):1245–1251. - PubMed
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischaemic stroke. New England Journal of Medicine. 1995;333:1581–1587. - PubMed
- Thomalla G, Sobesky J, Köhrmann M, et al. Two tales: hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia—MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 hours. Stroke. 2007;38(2):313–318. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources