Exercise training and peripheral arterial disease - PubMed (original) (raw)
Review
Exercise training and peripheral arterial disease
Tara L Haas et al. Compr Physiol. 2012 Oct.
Abstract
Peripheral arterial disease (PAD) is a common vascular disease that reduces blood flow capacity to the legs of patients. PAD leads to exercise intolerance that can progress in severity to greatly limit mobility, and in advanced cases leads to frank ischemia with pain at rest. It is estimated that 12 to 15 million people in the United States are diagnosed with PAD, with a much larger population that is undiagnosed. The presence of PAD predicts a 50% to 1500% increase in morbidity and mortality, depending on severity. Treatment of patients with PAD is limited to modification of cardiovascular disease risk factors, pharmacological intervention, surgery, and exercise therapy. Extended exercise programs that involve walking approximately five times per week, at a significant intensity that requires frequent rest periods, are most significant. Preclinical studies and virtually all clinical trials demonstrate the benefits of exercise therapy, including improved walking tolerance, modified inflammatory/hemostatic markers, enhanced vasoresponsiveness, adaptations within the limb (angiogenesis, arteriogenesis, and mitochondrial synthesis) that enhance oxygen delivery and metabolic responses, potentially delayed progression of the disease, enhanced quality of life indices, and extended longevity. A synthesis is provided as to how these adaptations can develop in the context of our current state of knowledge and events known to be orchestrated by exercise. The benefits are so compelling that exercise prescription should be an essential option presented to patients with PAD in the absence of contraindications. Obviously, selecting for a lifestyle pattern that includes enhanced physical activity prior to the advance of PAD limitations is the most desirable and beneficial.
© 2012 American Physiological Society
Figures
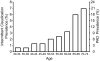
Figure 1
Prevalence of peripheral arterial disease, and the subset of patients with intermittent claudication, increases markedly with age. Reproduced from Norgren et al., (661) with permission.
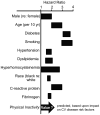
Figure 2
The risk factors for peripheral arterial disease are numerous, as illustrated by these hazard ratios. Figure adapted from Norgren et al., (661), with permission, and added concept from Booth et al., (80).
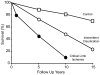
Figure 3
The increase in mortality with peripheral arterial disease is related to its severity. Reproduced from Norgren et al., (661) with permission.
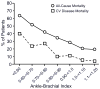
Figure 4
The increased mortality of peripheral arterial disease is predicted by the decline in the ankle-brachial artery pressure ratio. Reproduced from Resnick et al., (749) with permission.
Figure 5
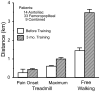
Typical increase in exercise tolerance, measured during a defined treadmill protocol and during free-pace walking, that was observed in patients with peripheral arterial disease who participated in an exercise program. Data taken from Carter et al., (132).
Figure 6
The predominance of angiogenic factors that are induced in response to repeated exercise (upper panel) and the combination of angiogenic, inflammatory and angiostatic factors that are prevalent during muscle ischemia (lower panel). Refer to the text for additional details.
Figure 7
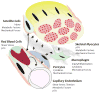
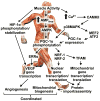
Activating stimuli and cellular interactions within the skeletal muscle microenvironment. Arrows denote paracrine signaling crosstalk that ensures co-ordination of the processes of angiogenesis, satellite cell activation and myocyte metabolic adaptation in response to physical/mechanical or biochemical stimuli.
Figure 8
An overview of signaling pathways that coordination exercise-induced angiogenesis and mitochondrial biogenesis. Information obtained from (12,31,403, 413, 513, 750, 1009).
Figure 9
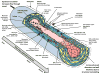
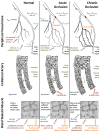
Summary of some key events in the remodeling of a collateral artery in response to upstream occlusion. The approximate time course is shown moving from upper left (with events occurring within hours/days of occlusion) to the bottom right (completion of remodeling after >1 month). Increased shear stress and vessel stretch following upstream occlusion leads to endothelial cell activation, adhesion molecule expression, and monocyte infiltration, followed by reorganization of the extracellular matrix. Phenotypic shift, migration, and proliferation of vascular smooth muscle cells leads to neointima formation and an increase in the number of smooth muscle cell layers. The process is complete when vascular smooth muscle cells have returned to a contractile phenotype and the vessel structure has regained a relatively normal appearance. (Not all cell types are shown at each time point, and the number of smooth muscle cell layers is limited for clarity).
Figure 10
Diagram of forces acting on peripheral collateral vasculature and the resulting changes in collateral-dependent blood flow in response to upstream arterial occlusion. Top: simplified representation of the peripheral vasculature. Collateral vessels are present under normal conditions (left). However, there is no pressure gradient across the collaterals. Moreover, collateral resistance is high due to the narrow vessel diameter. Thus, collateral blood flow is low under normal conditions. Following an acute occlusion (center), a pressure gradient is created across the collaterals, driving flow through the vessels. Vasodilation produces a further limited increase in collateral blood flow. Since the vessel diameter remains relatively small and the pressure gradient for flow is large, shear stress levels in the collaterals are high. High shear stress initiates structural remodeling, which is evident following chronic occlusion (right). Smooth muscle cell proliferation occurs, resulting in increased vascular wall thickness. Since the ends of the vessel are fixed, vascular growth also produces an increase in tortuosity of the collaterals. Eventually, the diameter of the vessel increases to a point where shear stress is reduced to non-stimulatory levels, and remodeling ceases. Middle: the events described above, seen at the level of the individual collateral artery. A limited number of smooth muscle layers is shown for clarity. Bottom: functional consequences of arterial occlusion and collateral remodeling in skeletal muscle of the distal limb. Vasodilation of collaterals following acute occlusion may provide sufficient flow for tissue needs under resting conditions depending on the location of the occlusion (center), but is insufficient for active skeletal muscle demands. Thus, distal skeletal muscle is at risk of ischemia and may become hypoxic. (The area of collateral remodeling in the proximal limb is itself well-perfused and non-hypoxic). Reduced tissue pO2 leads to opening of capillaries within the muscle. After structural remodeling of the collateral vasculature (right), blood flow capacity to the distal limb is improved and may suffice to support the demands of active skeletal muscle. In conjunction with arteriogenesis in the proximal limb, capillary proliferation (angiogenesis) occurs in distal tissue, in response to hypoxia and other factors.
Figure 11
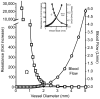
Relationships between vessel size and blood flow (right axis) and resistance (left axis) for a typical femoral artery of 5 mm diameter. Note the precipitous decline in blood flow, and increase in vascular resistance, as vessel caliber decreases, since these are a 4th-power function of vessel radius. Thus, blood flow capacity is only ~6% of normal, if the size of the vessel declines to one-half. The insert is an expanded region of interest.
Figure 12
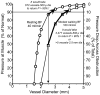
Calculated pressure to the distal calf muscles as a function of the reduction in caliber of the upstream vessel when blood flow to the distal limb is sufficient for resting tissue needs of 40 ml/min (circles) or during walking at a slow pace where blood flow needs increase to 160 ml/min (squares). Note that a reduction in upstream vessel caliber to one-half initial leads to a reduction in distal pressure to < 90% normal, a value that defines the presence of PAD. At the same time, this individual would experience a marked reduction in distal perfusion pressure to <50% of normal during walking. Note that it would take the development of ~3500 500μ or 5 2.5 mm diameter collateral vessels to recover distal perfusion pressure to above 90% during the mild walking rate.
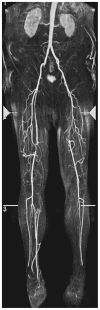
Figure 13
Magnetic Resonance angiograph illustrating that collateral vessels can develop to circumvent a short-segment occlusion (right superficial femoral artery) and long-segment occlusion (left femoral artery) of patients with PAD. Reproduced from Esterhammer et al with permission from (249).
Figure 14
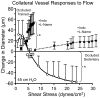
Influence of exercise training on the vasoresponsiveness of a collateral vessel as a function of shear stress. An initial modest dilatation to low shear stress in control animals (open circles) reverted to a dominant vasoconstriction at high shear stress. This response was eliminated in the presence of indomethacin, L-NAME, and in combination, as illustrated (filled circles), to a modest vasodilatation at very high shear stress. In contrast, collateral vessels from trained animals exhibited a marked vasodilation in the presence of indomethacin, L-NAME, and in combination, as illustrated (filled squares). This implies that exercise training induces a cyclooxygenase- and NOS-independent stimulus for vasodilatation. Data taken from Colleran et al (170) with permission.
Figure 15
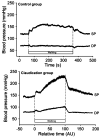
Example of hypertension during exercise in a group of patients with PAD who exhibit claudication. Note that the elevation in blood pressure in the claudicant group is greater than that of aged-matched control group well prior to the cessation of walking. Figure reproduced from Baake et al (47) with permission.
Similar articles
- Walking Exercise Therapy Effects on Lower Extremity Skeletal Muscle in Peripheral Artery Disease.
McDermott MM, Dayanidhi S, Kosmac K, Saini S, Slysz J, Leeuwenburgh C, Hartnell L, Sufit R, Ferrucci L. McDermott MM, et al. Circ Res. 2021 Jun 11;128(12):1851-1867. doi: 10.1161/CIRCRESAHA.121.318242. Epub 2021 Jun 10. Circ Res. 2021. PMID: 34110902 Review. - Optimising exercise training in peripheral arterial disease.
Bulmer AC, Coombes JS. Bulmer AC, et al. Sports Med. 2004;34(14):983-1003. doi: 10.2165/00007256-200434140-00004. Sports Med. 2004. PMID: 15571429 Review. - Vasoresponsiveness of collateral vessels in the rat hindlimb: influence of training.
Colleran PN, Li Z, Yang HT, Laughlin MH, Terjung RL. Colleran PN, et al. J Physiol. 2010 Apr 15;588(Pt 8):1293-307. doi: 10.1113/jphysiol.2009.186247. Epub 2010 Mar 1. J Physiol. 2010. PMID: 20194126 Free PMC article. - Cardiovascular training effect associated with polestriding exercise in patients with peripheral arterial disease.
Collins EG, Langbein WE, Orebaugh C, Bammert C, Hanson K, Reda D, Edwards LC, Littooy FN. Collins EG, et al. J Cardiovasc Nurs. 2005 May-Jun;20(3):177-85. doi: 10.1097/00005082-200505000-00009. J Cardiovasc Nurs. 2005. PMID: 15870588 Clinical Trial. - Peripheral artery disease: therapeutic advances.
Shamoun F, Sural N, Abela G. Shamoun F, et al. Expert Rev Cardiovasc Ther. 2008 Apr;6(4):539-53. doi: 10.1586/14779072.6.4.539. Expert Rev Cardiovasc Ther. 2008. PMID: 18402543 Review.
Cited by
- Diffusion tensor imaging indices of acute muscle damage are augmented after exercise in peripheral arterial disease.
Stavres J, Wang J, Sica CT, Blaha C, Herr M, Pai S, Cauffman A, Vesek J, Yang QX, Sinoway LI. Stavres J, et al. Eur J Appl Physiol. 2021 Sep;121(9):2595-2606. doi: 10.1007/s00421-021-04711-7. Epub 2021 Jun 9. Eur J Appl Physiol. 2021. PMID: 34106324 Free PMC article. - Metabolic regulation of exercise-induced angiogenesis.
Gorski T, De Bock K. Gorski T, et al. Vasc Biol. 2019 Mar 11;1(1):H1-H8. doi: 10.1530/VB-19-0008. eCollection 2019. Vasc Biol. 2019. PMID: 32923947 Free PMC article. Review. - Joint Angle Variability Is Altered in Patients with Peripheral Artery Disease after Six Months of Exercise Intervention.
Fallahtafti F, Salamifar Z, Hassan M, Rahman H, Pipinos I, Myers SA. Fallahtafti F, et al. Entropy (Basel). 2022 Oct 6;24(10):1422. doi: 10.3390/e24101422. Entropy (Basel). 2022. PMID: 37420442 Free PMC article. - Considerations for Implementation of an Ankle-Foot Orthosis to Improve Mobility in Peripheral Artery Disease.
Bashir AZ, Dinkel DM, Bapat GM, Despiegelaere H, Hassan M, Johanning JM, Pipinos II, Myers SA. Bashir AZ, et al. Arch Rehabil Res Clin Transl. 2021 Jan 5;3(1):100092. doi: 10.1016/j.arrct.2020.100092. eCollection 2021 Mar. Arch Rehabil Res Clin Transl. 2021. PMID: 33778468 Free PMC article. - The Role of Physiotherapy in Peripheral Artery Disease in Patients With Diabetes Mellitus: A Narrative Review.
Herrera D, Rueda Capistrani DE, Obando Vera S, Sanchez Cruz C, Linarez Nuñez KA, Banegas D, Argueta A, Murillo Md MI, Clervil K, Perez Moreno EJ, Calderon Martinez E. Herrera D, et al. Cureus. 2024 Jan 10;16(1):e52019. doi: 10.7759/cureus.52019. eCollection 2024 Jan. Cureus. 2024. PMID: 38344599 Free PMC article. Review.
References
- Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, Ergin Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–2242. - PubMed
- Abou-Khalil R, Le GF, Pallafacchina G, Valable S, Authier FJ, Rudnicki MA, Gherardi RK, Germain S, Chretien F, Sotiropoulos A, Lafuste P, Montarras D, Chazaud B. Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal. Cell Stem Cell. 2009;5:298–309. - PMC - PubMed
- Abumiya T, Sasaguri T, Taba Y, Miwa Y, Miyagi M. Shear Stress Induces Expression of Vascular Endothelial Growth Factor Receptor Flk-1/KDR Through the CT-Rich Sp1 Binding Site. Arterioscler Thromb Vasc Biol. 2002;22:907–913. - PubMed
Further Reading
- Schaper W, Schaper J. Arteriogenesis. Boston: Kluwer Academic Publishers; 2004. pp. 1–377.
- Charkravarthy MV, Booth FW. Exercise. Philadelphia: Hanley & Belfus; 2003. pp. 1–326.
Publication types
MeSH terms
Grants and funding
- R01 HL084494/HL/NHLBI NIH HHS/United States
- R37 AR021617/AR/NIAMS NIH HHS/United States
- T32 AR048523/AR/NIAMS NIH HHS/United States
- R37-AR21617/AR/NIAMS NIH HHS/United States
- F32-HL10406/HL/NHLBI NIH HHS/United States
- F32 HL010406/HL/NHLBI NIH HHS/United States
- R01 HL037387/HL/NHLBI NIH HHS/United States
- F32-HL10485/HL/NHLBI NIH HHS/United States
- R01-HL084494/HL/NHLBI NIH HHS/United States
- R01-HL37387/HL/NHLBI NIH HHS/United States
- P01-HL52490/HL/NHLBI NIH HHS/United States
- T32-AR048523/AR/NIAMS NIH HHS/United States
- F32 HL010485/HL/NHLBI NIH HHS/United States
- P01 HL052490/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources