Compound heterozygous mutations in SLC30A2/ZnT2 results in low milk zinc concentrations: a novel mechanism for zinc deficiency in a breast-fed infant - PubMed (original) (raw)
Case Reports
Compound heterozygous mutations in SLC30A2/ZnT2 results in low milk zinc concentrations: a novel mechanism for zinc deficiency in a breast-fed infant
Naoya Itsumura et al. PLoS One. 2013.
Abstract
Zinc concentrations in breast milk are considerably higher than those of the maternal serum, to meet the infant's requirements for normal growth and development. Thus, effective mechanisms ensuring secretion of large amounts of zinc into the milk operate in mammary epithelial cells during lactation. ZnT2 was recently found to play an essential role in the secretion of zinc into milk. Heterozygous mutations of human ZnT2 (hZnT2), including H54R and G87R, in mothers result in low (>75% reduction) secretion of zinc into the breast milk, and infants fed on the milk develop transient neonatal zinc deficiency. We identified two novel missense mutations in the SLC30A2/ZnT2 gene in a Japanese mother with low milk zinc concentrations (>90% reduction) whose infant developed severe zinc deficiency; a T to C transition (c.454T>C) at exon 4, which substitutes a tryptophan residue with an arginine residue (W152R), and a C to T transition (c.887C>T) at exon 7, which substitutes a serine residue with a leucine residue (S296L). Biochemical characterization using zinc-sensitive DT40 cells indicated that the W152R mutation abolished the abilities to transport zinc and to form a dimer complex, indicating a loss-of-function mutation. The S296L mutation retained both abilities but was extremely destabilized. The two mutations were found on different alleles, indicating that the genotype of the mother with low milk zinc was compound heterozygous. These results show novel compound heterozygous mutations in the SLC30A2/ZnT2 gene causing zinc deficiency in a breast-fed infant.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
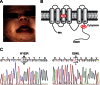
Figure 1. Identification of two missense mutations in the SLC30A2/ZnT2 gene in the mother of a zinc-deficient infant.
(A) Photograph of an affected infant with severe zinc deficiency. The dermatitis was erythematous and erosive, particularly around the infant's mouth. (B) Predicted topology of hZnT2 indicating the positions of the W152R and S296L substitutions found in this study. (C) Electropherograms showing SLC30A2/ZnT2 mutations in the affected mother. W152R and S296L mutations were found at exons 4 and 7 on different alleles.
Figure 2. Sequence alignment among hZnT2, hZnT3 and hZnT8.
The positions of tryptophan (corresponding to W152 in hZnT2) and serine (S296) residues (indicated by black arrowheads) identified in the affected mother with low milk zinc are completely conserved in hZnT3 and semi-conserved in hZnT8. The positions of histidine (corresponding to H54 in hZnT2) and glycine (G87) residues that have been identified are also indicated by gray arrowheads. Identical amino acids are indicated by *. The putative transmembrane regions, which are predicted by SOSUI (
http://bp.nuap.nagoya-u.ac.jp/sosui/
) using hZnT2 sequence, are shaded in gray.
Figure 3. No mutations were found in and around the promoter region of the SLC30A2/ZnT2 gene.
Alignment of the sequences of the mother with low milk zinc (“mother”) and the human genomic sequence deposited in the GenBank database (“human”). To show high homology in this region among mammals, where the MRE is completely conserved, the sequences are also aligned with those of rats and mice deposited in the GenBank database (corresponding regions from −110 to +22 of mouse Znt2 are shown. The transcription start site is indicated by gray shading). Identical nucleotides are indicated by * and the MRE sequence is indicated with bold letters.
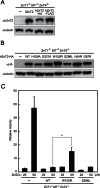
Figure 4. W152R hZnT2 loses the ability to transport zinc, but the S296L hZnT2 does not.
(A) The carboxyl-terminal epitope tags do not interfere with hZnT2 expression. Untagged and HA- or FLAG-tagged hZnT2 were stably expressed in ZnT1 −/− MT −/− ZnT4 −/− cells. Immunoblotting was performed using anti-hZnT2 antibody. (B) Confirmation of stable expression of the WT hZnT2-HA, W152R, S296L and other mutants of hZnT2-HA in ZnT1 −/− MT −/− ZnT4 −/− cells. Immunoblotting was performed using an anti-HA antibody. In both (A) and (B), 20 µg of total cellular protein was loaded onto each lane, and the same membrane was used for detection of both hZnT2 and tubulin. Tubulin is shown as a loading control. (C) Effects of zinc on MT-luciferase reporter gene expression in ZnT1 −/− MT −/− ZnT4 −/− cells stably expressing WT hZnT2-HA, W152R or S296L mutant hZnT2-HA. Relative activity of Luc is shown (the luciferase activity of ZnT1 −/− MT −/− ZnT4 −/− cells cultured without ZnSO4 is defined as 1). Each value is the mean ± SD of triplicate experiments. * denotes a significant difference of relative activity of Luc between the cells expressing WT and W152R mutant hZnT2 (P<0.05).
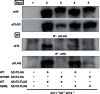
Figure 5. W152R hZnT2 mutant is not dominant negative because it fails to form functional dimers.
Tagged-hZnT2 WT or mutants were immunoprecipitated (IP) with antibodies against either the FLAG or HA epitopes. The immunoprecipitates were analyzed by immunoblotting using antibodies against the FLAG or HA tags. To estimate the amount of tagged hZnT2 WT and mutant proteins, 10% of each aliquot was subjected to immunoblot analysis (input panels). The IP experiments were performed four times, which gave the same results. The panels show the representative results.
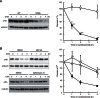
Figure 6. S296L mutation causes hZnT2 destabilized.
(A) The expression level of the hZnT2 protein at each time point. The ZnT1 −/− MT −/− ZnT4 −/− cells expressing WT hZnT2 or S296L mutant were treated with CHX and collected periodically over 4 h. Immunoblot analysis was performed to monitor hZnT2 levels (left panel). The band intensities of hZnT2 protein (○, WT; •, S296L mutant) are shown as the percentage of the intensity at 0 h (T0) after normalized by that of tubulin at each time (right panel). * and ** denote a significant difference between expression levels of the WT and S296L mutant hZnT2 at each time point (* P<0.05, ** P<0.01) (B) Lysosome inhibitor bafilomycin A1 and proteasome inhibitor MG132 block the degradation of S296L hZnT2 mutant. Immunoblot analysis (left panel) and the band intensities of hZnT2 protein (○, MG132; Δ, bafilomycin A1; • no inhibitor, right panel) are shown. In the right panels of both (A) and (B), each value is the mean ± SD of triplicate experiments. The same membrane was used for detection of both hZnT2 and tubulin. Tubulin is shown as a loading control. * and ** denote significant differences between expression levels in the absence and presence of bafilomycin A1 or MG132 at each time point (* P<0.05, ** P<0.01).
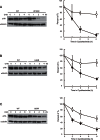
Figure 7. Protein stability of W152R, H54R and G87R hZnT2 mutants.
The expression levels of the WT (○) and W152R (•) mutant hZnT2 proteins (A), the WT (○) and H54R (•) mutant hZnT2 proteins (B), and the WT (○) and G87R (•) mutant hZnT2 proteins (C) at each time point. Immunoblot analysis was performed to monitor hZnT2 levels (left panel), as described in Figure 6. In the right panels of (A) – (C), each value is the mean ± SD of triplicate experiments. The same membrane was used for detection of both hZnT2 and tubulin. Tubulin is shown as a loading control. * and ** denote a significant difference between expression levels of the WT and W152R, H54R or G87R mutant hZnT2 at each time point (* P<0.05, ** P<0.01).
Similar articles
- Novel mutations in SLC30A2 involved in the pathogenesis of transient neonatal zinc deficiency.
Itsumura N, Kibihara Y, Fukue K, Miyata A, Fukushima K, Tamagawa-Mineoka R, Katoh N, Nishito Y, Ishida R, Narita H, Kodama H, Kambe T. Itsumura N, et al. Pediatr Res. 2016 Oct;80(4):586-94. doi: 10.1038/pr.2016.108. Epub 2016 May 16. Pediatr Res. 2016. PMID: 27304099 - Exome Sequencing of SLC30A2 Identifies Novel Loss- and Gain-of-Function Variants Associated with Breast Cell Dysfunction.
Alam S, Hennigar SR, Gallagher C, Soybel DI, Kelleher SL. Alam S, et al. J Mammary Gland Biol Neoplasia. 2015 Dec;20(3-4):159-72. doi: 10.1007/s10911-015-9338-z. Epub 2015 Aug 21. J Mammary Gland Biol Neoplasia. 2015. PMID: 26293594 - High proportion of transient neonatal zinc deficiency causing alleles in the general population.
Golan Y, Lehvy A, Horev G, Assaraf YG. Golan Y, et al. J Cell Mol Med. 2019 Feb;23(2):828-840. doi: 10.1111/jcmm.13982. Epub 2018 Nov 18. J Cell Mol Med. 2019. PMID: 30450693 Free PMC article. - The role of the zinc transporter SLC30A2/ZnT2 in transient neonatal zinc deficiency.
Golan Y, Kambe T, Assaraf YG. Golan Y, et al. Metallomics. 2017 Oct 18;9(10):1352-1366. doi: 10.1039/c7mt00162b. Metallomics. 2017. PMID: 28665435 Review. - Genetic causes and gene–nutrient interactions in mammalian zinc deficiencies: acrodermatitis enteropathica and transient neonatal zinc deficiency as examples.
Kasana S, Din J, Maret W. Kasana S, et al. J Trace Elem Med Biol. 2015 Jan;29:47-62. doi: 10.1016/j.jtemb.2014.10.003. J Trace Elem Med Biol. 2015. PMID: 25468189 Review.
Cited by
- Essential Role for Zinc Transporter 2 (ZnT2)-mediated Zinc Transport in Mammary Gland Development and Function during Lactation.
Lee S, Hennigar SR, Alam S, Nishida K, Kelleher SL. Lee S, et al. J Biol Chem. 2015 May 22;290(21):13064-78. doi: 10.1074/jbc.M115.637439. Epub 2015 Apr 7. J Biol Chem. 2015. PMID: 25851903 Free PMC article. - Biological underpinnings of breastfeeding challenges: the role of genetics, diet, and environment on lactation physiology.
Lee S, Kelleher SL. Lee S, et al. Am J Physiol Endocrinol Metab. 2016 Aug 1;311(2):E405-22. doi: 10.1152/ajpendo.00495.2015. Epub 2016 Jun 28. Am J Physiol Endocrinol Metab. 2016. PMID: 27354238 Free PMC article. Review. - Demonstrating aspects of multiscale modeling by studying the permeation pathway of the human ZnT2 zinc transporter.
Golan Y, Alhadeff R, Glaser F, Ganoth A, Warshel A, Assaraf YG. Golan Y, et al. PLoS Comput Biol. 2018 Nov 2;14(11):e1006503. doi: 10.1371/journal.pcbi.1006503. eCollection 2018 Nov. PLoS Comput Biol. 2018. PMID: 30388104 Free PMC article. - In silico mapping of quantitative trait loci (QTL) regulating the milk ionome in mice identifies a milk iron locus on chromosome 1.
Hadsell DL, Hadsell LA, Rijnkels M, Carcamo-Bahena Y, Wei J, Williamson P, Grusak MA. Hadsell DL, et al. Mamm Genome. 2018 Oct;29(9-10):632-655. doi: 10.1007/s00335-018-9762-7. Epub 2018 Aug 2. Mamm Genome. 2018. PMID: 30073618 - Paradoxical zinc toxicity and oxidative stress in the mammary gland during marginal dietary zinc deficiency.
Bostanci Z, Mack RP Jr, Lee S, Soybel DI, Kelleher SL. Bostanci Z, et al. Reprod Toxicol. 2015 Jul;54:84-92. doi: 10.1016/j.reprotox.2014.07.076. Epub 2014 Aug 1. Reprod Toxicol. 2015. PMID: 25088245 Free PMC article.
References
- Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73: 79–118. - PubMed
- Maret W, Li Y (2009) Coordination dynamics of zinc in proteins. Chem Rev 109: 4682–4707. - PubMed
- Fukada T, Kambe T (2011) Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 3: 662–674. - PubMed
- Prasad AS (1995) Zinc: An overview. Nutrition 11: 93–99. - PubMed
- Hambidge M (2000) Human zinc deficiency. J Nutr 130: 1344S–1349S. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by the Mochida Memorial Foundation for Medical and Pharmaceutical Research; the Suzuken Memorial Foundation (to TK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources