Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion - PubMed (original) (raw)
Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion
Hariom Yadav et al. J Biol Chem. 2013.
Abstract
Obesity and diabetes are associated with excess caloric intake and reduced energy expenditure resulting in a negative energy balance. The incidence of diabetes has reached epidemic proportions, and childhood diabetes and obesity are increasing alarmingly. Therefore, it is important to develop safe, easily deliverable, and economically viable treatment alternatives for these diseases. Here, we provide data supporting the candidacy of probiotics as such a therapeutic modality against obesity and diabetes. Probiotics are live bacteria that colonize the gastrointestinal tract and impart beneficial effects for health. However, their widespread prescription as medical therapies is limited primarily because of the paucity of our understanding of their mechanism of action. Here, we demonstrate that the administration of a probiotic, VSL#3, prevented and treated obesity and diabetes in several mouse models. VSL#3 suppressed body weight gain and insulin resistance via modulation of the gut flora composition. VSL#3 promoted the release of the hormone GLP-1, resulting in reduced food intake and improved glucose tolerance. The VSL#3-induced changes were associated with an increase in the levels of a short chain fatty acid (SCFA), butyrate. Using a cell culture system, we demonstrate that butyrate stimulated the release of GLP-1 from intestinal L-cells, thereby providing a plausible mechanism for VSL#3 action. These findings suggest that probiotics such as VSL#3 can modulate the gut microbiota-SCFA-hormone axis. Moreover, our results indicate that probiotics are of potential therapeutic utility to counter obesity and diabetes.
Keywords: Diabetes; GLP-1; Gut Flora; Leptin; Metabolism; Obesity; Probiotics.
Figures
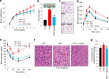
FIGURE 1.
VSL#3 prevented high fat diet-induced obesity and diabetes. a and b, VSL#3 administration reduced body weight gain (a) and fat mass (b) in HFD-fed mice. HFD+VSL#3-fed mice maintained body weight and fat mass similar to LFD-fed mice. c, adipocyte size is smaller in HFD+VSL#3-fed mice than in HFD-fed control mice. d and e, VSL#3 treatment in HFD-fed mice enhanced glucose homeostasis as shown by improved glucose tolerance tests (d) and insulin tolerance tests (e). f, VSL#3 administration in HFD-fed mice dramatically reduced hepatic steatosis in comparison with non-treated control HFD mice (fat droplets indicated by red arrows) and maintained liver morphology similar to LFD-fed mice. g, VSL#3-fed HFD mice exhibited reduced food intake. Values presented here represent the mean ± S.E. for each group. Values indicated with asterisks are significantly different at the level of: *, p < 0.05; **, p < 0.001; and ***, p < 0.0001. Values indicated with hash marks are significantly different at the level of: #, p < 0.05; ##, p < 0.001; and ###, p < 0.0001 from HFD-fed animals.
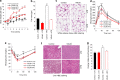
FIGURE 2.
VSL#3 reversed obesity and diabetes in HFD-fed mice. a and b, VSL#3 administration in DIO mice suppressed body weight gain (a) and fat mass (b). Beneficial effects of VSL#3 were also seen in mice switched to LFD. c, administration of VSL#3 reduced adipocyte size in white adipose tissue. d and e, glucose tolerance (d) and insulin tolerance (e) were significantly enhanced in VSL#3-treated DIO mice. f, hepatic steatosis was improved in VSL#3-treated DIO mice in both HFD- and LFD-fed groups as compared with their corresponding controls. g, VSL#3 treatment also decreased food intake in DIO mice. The values presented here represent the mean ± S.E. for each group. Values indicated with asterisks are significantly different at the level of: *, p < 0.05; **, p < 0.001; and ***, p < 0.0001.
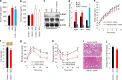
FIGURE 3.
Leptin levels in VSL#3-administered mice and effects of VSL#3 on obesity and diabetes in Lepob/ob mice. a and b, leptin levels upon VSL#3 treatment in the preventive (a) and therapeutic model (b). c, VSL#3 enhanced Stat3 phosphorylation in the hypothalamus of HFD-fed mice in comparison with HFD control mice. d, expression levels of food intake regulatory genes, i.e. AgRP, NpY, and POMC, were significantly modulated in the hypothalamus of VSL#3-treated mice compared with their control mice. e and f, Lepob/ob mice administered VSL#3 exhibited a significant reduction in body weight gain (e) and fat mass (f) compared with Lepob/ob mice not fed VSL#3. g and h, improved glucose tolerance tests (g) and insulin tolerance tests (h) in VSL#3-treated Lepob/ob mice compared with Lepob/ob mice not fed VSL#3. i, hepatic fat accumulation was substantially decreased in VSL#3-treated Lepob/ob mice. j, VSL#3-fed Lepob/ob mice exhibited decreased food intake. The values presented here represent the mean ± S.E. for each group. Values indicated with asterisks are significantly different at the level of: *, p < 0.05; **, p < 0.001; and ***, p < 0.0001.
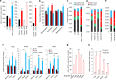
FIGURE 4.
VSL#3 altered gut flora composition and increased butyrate and GLP-1 levels. a, VSL#3 feeding dramatically increased serum GLP-1 levels in the three mouse models (preventive, therapeutic, and Lepob/ob mice). b, specific bacterial abundance, i.e. Firmicutes, Bacteriodetes, lactobacilli, and bifidobacteria, was significantly changed upon VSL#3 treatment. Abundance of bacteria in mice on either LFD or HFD, with or without VSL#3, is shown (black, LFD; red, HFD; light blue, HFD + VSL3). c–e, VSL#3 administration significantly increased butyrate levels in fecal samples of HFD-fed mice (c and d) and in Lepob/ob mice (e). f, genes implicated in GLP-1 synthesis and secretion (i.e. Gcg, Pcsk1, and Slc5a1) and butyrate-responsive gene (i.e. Ffar3) were significantly increased in different parts of the intestine from VSL#3-fed mice. g and h, butyrate treatment of NCI-H716 cells significantly increased GLP-1 secretion in a dose-dependent manner (g) and increased GLP-1 synthesis and secretion and butyrate-responsive gene expression (h). The values presented here represent the mean ± S.E. for each group. Values indicated with asterisks are significantly different at the level of: *, p < 0.05; **, p < 0.001; and ***, p < 0.0001.
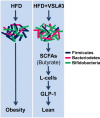
FIGURE 5.
Proposed mechanism of action of VSL#3 against obesity and diabetes. VSL#3 feeding significantly changed the composition of the gut flora, i.e. decreased Firmicutes (dark blue rods) and increased Bacteriodetes (red rods) and bifidobacteria (green rods). This change in the microbiota is associated with increased butyrate production. Butyrate further increased GLP-1 secretion from intestinal L-cells that ultimately enhanced metabolic function to prevent obesity and diabetes in the three mouse models studied.
References
- Kim M. S., Lee M. S., Kown D. Y. (2011) Inflammation-mediated obesity and insulin resistance as targets for nutraceuticals. Ann. N.Y. Acad. Sci. 1229, 140–146 - PubMed
- Lin C. Y., Chen P. C., Kuo H. K., Lin L. Y., Lin J. W., Hwang J. J. (2010) Effects of obesity, physical activity, and cardiorespiratory fitness on blood pressure, inflammation, and insulin resistance in the National Health and Nutrition Survey 1999–2002. Nutr. Metab. Cardiovasc. Dis. 20, 713–719 - PubMed
- Esteve E., Ricart W., Fernández-Real J. M. (2011) Gut microbiota interactions with obesity, insulin resistance and type 2 diabetes: did gut microbiote co-evolve with insulin resistance? Curr. Opin. Clin. Nutr. Metab. Care 14, 483–490 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical