Glycoprotein non-metastatic b (GPNMB): A metastatic mediator and emerging therapeutic target in cancer - PubMed (original) (raw)
Glycoprotein non-metastatic b (GPNMB): A metastatic mediator and emerging therapeutic target in cancer
Gordana Maric et al. Onco Targets Ther. 2013.
Abstract
Molecularly targeted therapies are rapidly growing with respect to their clinical development and impact on cancer treatment due to their highly selective anti-tumor action. However, many aggressive cancers such as triple-negative breast cancer (TNBC) currently lack well-defined therapeutic targets against which such agents can be developed. The identification of tumor-associated antigens and the generation of antibody drug-conjugates represent an emerging area of intense interest and growth in the field of cancer therapeutics. Glycoprotein non-metastatic b (GPNMB) has recently been identified as a gene that is over-expressed in numerous cancers, including TNBC, and often correlates with the metastatic phenotype. In breast cancer, GPNMB expression in the tumor epithelium is associated with a reduction in disease-free and overall survival. Based on these findings, glembatumumab vedotin (CDX-011), an antibody-drug conjugate that selectively targets GPNMB, is currently being investigated in clinical trials for patients with metastatic breast cancer and unresectable melanoma. This review discusses the physiological and potential pathological roles of GPNMB in normal and cancer tissues, respectively, and details the clinical advances and challenges in targeting GPNMB-expressing malignancies.
Keywords: CDX-011; GPNMB; antibody-drug conjugates; breast cancer; osteoactivin.
Figures
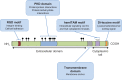
Figure 1
A schematic representation of GPNMB indicating the domains and motifs contributing to GPNMB function. Notes: The symbols (filled circles) located above the extracellular domain of GPNMB represent glycosylation sites. The RGD sequence comprises an integrin binding domain, where R = Arginine, G = Glycine, D = Aspartic acid. The YxxI sequence constitutes a hemITAM motif, where Y = tyrosine, x = any amino acid, I = isoleucine. The di-leucine motif is a lysosomal/endosomal targeting motif of the D/ExxxLL type, where D = Aspartic acid, E = Glutamic acid, x = any amino acid, L = leucine. Abbreviations: GPNMB, glycoprotein non-metastatic b; hemiTAM, immunoreceptor tyrosine-based activation motif; PKD, polycystic kidney disease; RGD, integrin-binding.
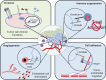
Figure 2
Potential mechanisms through which GPNMB promotes malignant cellular phenotypes within cancer cells. Notes: GPNMB may act cell autonomously (green panel) to induce intracellular signaling, which can influence the expression of multiple targets, including matrix metalloproteinases and cytokines, and enhance the invasiveness of tumor cells. GPNMB may also be important in regulating interactions between tumor cells and cells within the tumor microenvironment (blue panels). It can act as a cell/cell adhesion molecule by engaging integrins expressed on cells in the tumor microenvironment, such as endothelial cells. GPNMB-mediated interactions with syndecan-4 expressed on T cells can block the proliferation and activation of these cells, leading to an immunosuppressive environment favoring tumor growth. Finally, GPNMB may function in a paracrine fashion due to shedding of its extracellular domain, or through its release from cells in the form of microvesicles, leading to endothelial cell recruitment. All of these potential functions of GPNMB can promote tumor growth, invasion, and metastasis in a variety of cancer cells. Abbreviations: ECD, extracellular domain; GPNMB, glycoprotein non-metastatic b.
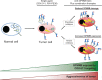
Figure 3
Therapeutic strategies employing anti-GPNMB antibody-drug conjugates (ADCs). Notes: in normal cells, GPNMB is preferentially localized within endosomal/lysosomal compartments, which is not accessible to anti-GPNMB ADCs. In many cancers, including breast, melanoma, and brain cancers, the levels of GPNMB expression increases and a greater proportion is localized on the cell surface. These GPNMB-expressing cancer cells are more susceptible to killing by anti-GPNMB ADCs (CDX-011, F6v-PE38). Evidence suggests that coupling kinase inhibitors (serine/threonine and tyrosine kinase inhibitors), which increase GPNMB expression, may enhance the efficacy of tumor cell killing by anti-GPNMB ADCs. Likewise, inhibiting GPNMB shedding could also lead to greater GPNMB surface expression and more targets for anti-GPNMB ADCs. Thus, GPNMB represents an attractive target due to low surface expression in normal cells and its increased expression in cancer cells, which leads to better tumor cell killing with anti-GPNMB ADCs. Combination therapies have the potential to achieve benefit from enhanced efficacy of the anti-GPNMB ADCs and effects of the coupled inhibitors (kinase inhibitors), but there is the potential risk that those tumor cells not killed by combination treatment may adopt increasing malignant phenotypes due to elevated GPNMB expression. Abbreviations: ADC, antibody-drug conjugate; CDX-011, glembatumumab vedotin; GPNMB, glycoprotein non-metastatic b.
Similar articles
- Glycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancer.
Rose AA, Grosset AA, Dong Z, Russo C, Macdonald PA, Bertos NR, St-Pierre Y, Simantov R, Hallett M, Park M, Gaboury L, Siegel PM. Rose AA, et al. Clin Cancer Res. 2010 Apr 1;16(7):2147-56. doi: 10.1158/1078-0432.CCR-09-1611. Epub 2010 Mar 9. Clin Cancer Res. 2010. PMID: 20215530 - EMERGE: A Randomized Phase II Study of the Antibody-Drug Conjugate Glembatumumab Vedotin in Advanced Glycoprotein NMB-Expressing Breast Cancer.
Yardley DA, Weaver R, Melisko ME, Saleh MN, Arena FP, Forero A, Cigler T, Stopeck A, Citrin D, Oliff I, Bechhold R, Loutfi R, Garcia AA, Cruickshank S, Crowley E, Green J, Hawthorne T, Yellin MJ, Davis TA, Vahdat LT. Yardley DA, et al. J Clin Oncol. 2015 May 10;33(14):1609-19. doi: 10.1200/JCO.2014.56.2959. Epub 2015 Apr 6. J Clin Oncol. 2015. PMID: 25847941 Clinical Trial. - Preclinical PET imaging of glycoprotein non-metastatic melanoma B in triple negative breast cancer: feasibility of an antibody-based companion diagnostic agent.
Marquez-Nostra BV, Lee S, Laforest R, Vitale L, Nie X, Hyrc K, Keler T, Hawthorne T, Hoog J, Li S, Dehdashti F, Ma CX, Lapi SE. Marquez-Nostra BV, et al. Oncotarget. 2017 Nov 1;8(61):104303-104314. doi: 10.18632/oncotarget.22228. eCollection 2017 Nov 28. Oncotarget. 2017. PMID: 29262642 Free PMC article. - Management of metastatic breast cancer with second-generation antibody-drug conjugates: focus on glembatumumab vedotin (CDX-011, CR011-vcMMAE).
Vaklavas C, Forero A. Vaklavas C, et al. BioDrugs. 2014 Jun;28(3):253-63. doi: 10.1007/s40259-014-0085-2. BioDrugs. 2014. PMID: 24496926 Review. - Targeting GPNMB with glembatumumab vedotin: Current developments and future opportunities for the treatment of cancer.
Rose AAN, Biondini M, Curiel R, Siegel PM. Rose AAN, et al. Pharmacol Ther. 2017 Nov;179:127-141. doi: 10.1016/j.pharmthera.2017.05.010. Epub 2017 May 22. Pharmacol Ther. 2017. PMID: 28546082 Review.
Cited by
- Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability.
Nguyen TD, Bordeau BM, Balthasar JP. Nguyen TD, et al. Cancers (Basel). 2023 Jan 24;15(3):713. doi: 10.3390/cancers15030713. Cancers (Basel). 2023. PMID: 36765668 Free PMC article. Review. - Advances in therapy for pediatric sarcomas.
Weiss A, Gill J, Goldberg J, Lagmay J, Spraker-Perlman H, Venkatramani R, Reed D. Weiss A, et al. Curr Oncol Rep. 2014;16(8):395. doi: 10.1007/s11912-014-0395-z. Curr Oncol Rep. 2014. PMID: 24894064 Review. - Quantitative trait locus mapping identifies the Gpnmb gene as a modifier of mouse macrophage lysosome function.
Robinet P, Ritchey B, Lorkowski SW, Alzayed AM, DeGeorgia S, Schodowski E, Traughber CA, Smith JD. Robinet P, et al. Sci Rep. 2021 May 13;11(1):10249. doi: 10.1038/s41598-021-89800-5. Sci Rep. 2021. PMID: 33986446 Free PMC article. - An Inducible Luminescent System to Explore Parkinson's Disease-Associated Genes.
Gandy A, Maussion G, Al-Habyan S, Nicouleau M, You Z, Chen CX, Abdian N, Aprahamian N, Krahn AI, Larocque L, Durcan TM, Deneault E. Gandy A, et al. Int J Mol Sci. 2024 Aug 31;25(17):9493. doi: 10.3390/ijms25179493. Int J Mol Sci. 2024. PMID: 39273438 Free PMC article. - Lymphotoxin-β promotes breast cancer bone metastasis colonization and osteolytic outgrowth.
Wang X, Zhang T, Zheng B, Lu Y, Liang Y, Xu G, Zhao L, Tao Y, Song Q, You H, Hu H, Li X, Sun K, Li T, Zhang Z, Wang J, Lan X, Pan D, Fu YX, Yue B, Zheng H. Wang X, et al. Nat Cell Biol. 2024 Sep;26(9):1597-1612. doi: 10.1038/s41556-024-01478-9. Epub 2024 Aug 15. Nat Cell Biol. 2024. PMID: 39147874
References
- Forouzanfar MH, Foreman KJ, Delossantos AM, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011;378(9801):1461–1484. - PubMed
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. - PubMed
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources