Molecular mechanisms and treatment of radiation-induced lung fibrosis - PubMed (original) (raw)
Review
Molecular mechanisms and treatment of radiation-induced lung fibrosis
Nian-Hua Ding et al. Curr Drug Targets. 2013 Oct.
Free PMC article
Abstract
Radiation-induced lung fibrosis (RILF) is a severe side effect of radiotherapy in lung cancer patients that presents as a progressive pulmonary injury combined with chronic inflammation and exaggerated organ repair. RILF is a major barrier to improving the cure rate and well-being of lung cancer patients because it limits the radiation dose that is required to effectively kill tumor cells and diminishes normal lung function. Although the exact mechanism is unclear, accumulating evidence suggests that various cells, cytokines and regulatory molecules are involved in the tissue reorganization and immune response modulation that occur in RILF. In this review, we will summarize the general symptoms, diagnostics, and current understanding of the cells and molecular factors that are linked to the signaling networks implicated in RILF. Potential approaches for the treatment of RILF will also be discussed. Elucidating the key molecular mediators that initiate and control the extent of RILF in response to therapeutic radiation may reveal additional targets for RILF treatment to significantly improve the efficacy of radiotherapy for lung cancer patients.
Figures
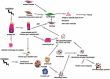
Fig. (1)
Numerous cell types are involved in RILF. Irradiation causes delayed damage to resident lung cells, leading primarily to the injury and apoptosis of bronchiolar epithelial cells. Via EMT, injured epithelial cells provide a source of myofibroblasts, which produce collagens (especially types I and III), fibronectins and other matrix molecules. Myofibroblasts can originate from resident fibroblasts and circulating fibroblast-like cells called fibrocytes, which are derived from bone marrow stem cells. The alveolar type II epithelial cells (AE2) in patients with RILF express high levels of EMT-associated protein markers, suggesting that the AE2 cells had acquired a mesenchymal phenotype. After lung injury, AE2 cells can convert to alveolar type I epithelial cells to re-establish a functional alveolar epithelium. Thorax irradiation can also trigger the recruitment of various immune cells into the lung, such as monocytic cells, neutrophils, basophils and lymphocytes, which are associated with the characteristic changes in the local and systemic expression of cytokines and chemokines.
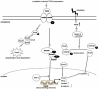
Fig. (2)
Signaling pathways in RILF. Irradiation induces TGF-β expression. Ligand binding to TGF-β activates type II TGF-β receptor serine/threonine kinases. TGF-β stimulation results in the induction of Smad proteins, which are transcription factors that also induce other transcription factors, including Slug, Snail, Scatter, lymphoid enhancing factor-1, and β-catenin. FoxM1 binds to and increases the promoter activity of the Snail1 gene. NRF2 binding to a Smad Binding Element (SBE) suppresses TGF-β target gene expression. TGF-β1 also activates non-Smad-mediated signaling pathways, which are associated with occludin at tight junctions, to phosphorylate Par6. Phosphorylated Par6 recruits Smurf1, resulting in the ubiquitination and degradation of RhoA, which is responsible for stress fiber formation and the maintenance of apical-basal polarity and junctional stability. Radiation activates the MEK/ERK signaling pathway by increasing ROS generation. Activated ERK1/2 phosphorylates GSK3β, resulting in its inactivation and leading to the disassociation of GSK3β and Snail. Unbound Snail then migrates to the nucleus and represses E-cadherin, leading to a mesenchymal-like phenotypic change.
Similar articles
- Quantitative assessment of radiation dose and fractionation effects on normal tissue by utilizing a novel lung fibrosis index model.
Zhou C, Jones B, Moustafa M, Schwager C, Bauer J, Yang B, Cao L, Jia M, Mairani A, Chen M, Chen L, Debus J, Abdollahi A. Zhou C, et al. Radiat Oncol. 2017 Nov 7;12(1):172. doi: 10.1186/s13014-017-0912-y. Radiat Oncol. 2017. PMID: 29116014 Free PMC article. - The features of radiation induced lung fibrosis related with dosimetric parameters.
Oh YT, Noh OK, Jang H, Chun M, Park KJ, Park KJ, Kim MH, Park HJ. Oh YT, et al. Radiother Oncol. 2012 Mar;102(3):343-6. doi: 10.1016/j.radonc.2012.02.003. Epub 2012 Feb 17. Radiother Oncol. 2012. PMID: 22342420 - Thalidomide (THD) alleviates radiation induced lung fibrosis (RILF) via down-regulation of TGF-β/Smad3 signaling pathway in an Nrf2-dependent manner.
Bian C, Qin WJ, Zhang CY, Zou GL, Zhu YZ, Chen J, Zhao R, Wang YY, Zhe H. Bian C, et al. Free Radic Biol Med. 2018 Dec;129:446-453. doi: 10.1016/j.freeradbiomed.2018.10.423. Epub 2018 Oct 16. Free Radic Biol Med. 2018. PMID: 30339882 - Radiation induced lung injury: prediction, assessment and management.
Giridhar P, Mallick S, Rath GK, Julka PK. Giridhar P, et al. Asian Pac J Cancer Prev. 2015;16(7):2613-7. doi: 10.7314/apjcp.2015.16.7.2613. Asian Pac J Cancer Prev. 2015. PMID: 25854336 Review. - Non-small cell lung cancer therapy-related pulmonary toxicity: an update on radiation pneumonitis and fibrosis.
Kong FM, Ten Haken R, Eisbruch A, Lawrence TS. Kong FM, et al. Semin Oncol. 2005 Apr;32(2 Suppl 3):S42-54. doi: 10.1053/j.seminoncol.2005.03.009. Semin Oncol. 2005. PMID: 16015535 Review.
Cited by
- The Complex Interplay of TGF-β and Notch Signaling in the Pathogenesis of Fibrosis.
Bakalenko N, Kuznetsova E, Malashicheva A. Bakalenko N, et al. Int J Mol Sci. 2024 Oct 8;25(19):10803. doi: 10.3390/ijms251910803. Int J Mol Sci. 2024. PMID: 39409132 Free PMC article. Review. - Ferroptosis-Associated Extracellular Matrix Remodeling in Radiation-Induced Lung Fibrosis Progression.
Yan X, Yang P, Yang C, Wang Y, Feng Z, Liu T, Li Y, Zhou C, Li M. Yan X, et al. Dose Response. 2024 Sep 28;22(3):15593258241289829. doi: 10.1177/15593258241289829. eCollection 2024 Jul-Sep. Dose Response. 2024. PMID: 39351078 Free PMC article. - Current Insights into Molecular Mechanisms and Potential Biomarkers for Treating Radiation-Induced Liver Damage.
Saha B, Pallatt S, Banerjee A, Banerjee AG, Pathak R, Pathak S. Saha B, et al. Cells. 2024 Sep 16;13(18):1560. doi: 10.3390/cells13181560. Cells. 2024. PMID: 39329744 Free PMC article. Review. - Feasibility, safety and preliminary efficacy of preoperative stereotactic radiotherapy on the future pancreatic neck transection margin to reduce the risk of pancreatic fistula after high-risk pancreatoduodenectomy (FIBROPANC): protocol for a multicentre, single-arm trial.
Suurmeijer JA, Wismans LV, Hendriks TE, Bruynzeel AM, Nuyttens JJ, Intven MPW, van Driel LMJW, Groot Koerkamp B, Busch OR, Stoker JJ, Verheij J, Farina A, Doukas M, de Hingh IHJ, Lips DJ, van der Harst E, van Tienhoven G, Besselink MG, van Eijck CHJ; Dutch Pancreatic Cancer Group. Suurmeijer JA, et al. BMJ Open. 2024 Sep 24;14(9):e087193. doi: 10.1136/bmjopen-2024-087193. BMJ Open. 2024. PMID: 39317507 Free PMC article. - Nintedanib Mitigates Radiation-Induced Pulmonary Fibrosis by Suppressing Epithelial Cell Inflammatory Response and Inhibiting Fibroblast-to-Myofibroblast Transition.
Tu J, Chen X, Li C, Liu C, Huang Y, Wang X, Liang H, Yuan X. Tu J, et al. Int J Biol Sci. 2024 Jun 11;20(9):3353-3371. doi: 10.7150/ijbs.92620. eCollection 2024. Int J Biol Sci. 2024. PMID: 38993568 Free PMC article.
References
- Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction. and prevention. Int J Radiat Oncol Biol Phys. 2005;63:5–24. - PubMed
- Ghafoori P, Marks LB, Vujaskovic Z, Kelsey CR. Radiation-induced lung injury.Assessent.management., and prevention. Oncology Williston Park discussion . 2008; 52 22(3 ):37–47. - PubMed
- McDonald S, Rubin P, Phillips TL, Marks LB. Injury to the lung from cancer therapy: clinical syndromes. measurable endponts.and potential scoring systems. Int J Radiat Oncol Biol Phys. 1995; 31:1187–203. - PubMed
- Fan M, Marks LB, Lind P, et al. Relating radiation-induced regional lung injury to changes in pulmonary function tests. Int J Radiat Oncol Biol Phys. 2001;51:311–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical