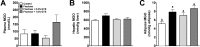Dietary fructose feeding increases adipose methylglyoxal accumulation in rats in association with low expression and activity of glyoxalase-2 - PubMed (original) (raw)
Dietary fructose feeding increases adipose methylglyoxal accumulation in rats in association with low expression and activity of glyoxalase-2
Christopher Masterjohn et al. Nutrients. 2013.
Abstract
Methylglyoxal is a precursor to advanced glycation endproducts that may contribute to diabetes and its cardiovascular-related complications. Methylglyoxal is successively catabolized to D-lactate by glyoxalase-1 and glyoxalase-2. The objective of this study was to determine whether dietary fructose and green tea extract (GTE) differentially regulate methylglyoxal accumulation in liver and adipose, mediated by tissue-specific differences in the glyoxalase system. We fed six week old male Sprague-Dawley rats a low-fructose diet (10% w/w) or a high-fructose diet (60% w/w) containing no GTE or GTE at 0.5% or 1.0% for nine weeks. Fructose-fed rats had higher (P < 0.05) adipose methylglyoxal, but GTE had no effect. Plasma and hepatic methylglyoxal were unaffected by fructose and GTE. Fructose and GTE also had no effect on the expression or activity of glyoxalase-1 and glyoxalase-2 at liver or adipose. Regardless of diet, adipose glyoxalase-2 activity was 10.8-times lower (P < 0.05) than adipose glyoxalase-1 activity and 5.9-times lower than liver glyoxalase-2 activity. Adipose glyoxalase-2 activity was also inversely related to adipose methylglyoxal (r = -0.61; P < 0.05). These findings suggest that fructose-mediated adipose methylglyoxal accumulation is independent of GTE supplementation and that its preferential accumulation in adipose compared to liver is due to low constitutive expression of glyoxalase-2.
Figures
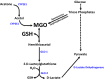
Figure 1
Major pathways of methylglyoxal (MGO) formation and detoxification. MGO in vivo is formed primarily from two pathways [2,24]. (Upper Right) triose phosphates derived from glycolysis spontaneously dephosphorylate to form MGO. (Upper Left) Acetone derived from ketogenesis is successively converted by cytochrome P450 2E1 (CYP2E1) to acetol and then to MGO. MGO detoxification (bottom left) occurs through the glutathione (GSH)-dependent glyoxalase (GLO) pathway [2]. MGO and GSH spontaneously form a hemithioacetal adduct that is successively detoxified by GLO-1 and GLO-2 to
d
-lactate, which is then converted to pyruvate by
d
-lactate dehydrogenase.
Figure 2
(a) MGO concentrations in plasma. (b) MGO concentrations in liver. (c) MGO concentrations in adipose. MGO concentrations are shown from rats fed a starch-based control diet containing 50% (w/w) starch and 10% (w/w) fructose (Control), a high-fructose diet containing 60% fructose (Fructose), the fructose diet containing 0.5% green tea extract (GTE) (w/w) (Fructose + 0.5% GTE), or the fructose diet containing 1.0% GTE (Fructose + 1.0% GTE) for nine weeks (means ± SE; n = 8–9/group). Samples were acidified with PCA, derivitized with OPD, and analyzed by HPLC-UV. 1-Way ANOVA main effects are significant for adipose (P < 0.01) but not plasma or liver (_P_ > 0.05). Group means without a common superscript are different (P < 0.05).
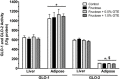
Figure 3
GLO-1 and GLO-2 activities in liver and adipose tissue. GLO-1 and GLO-2 activities are shown from rats fed a starch-based control diet containing 50% (w/w) starch and 10% (w/w) fructose (Control), a high-fructose diet containing 60% fructose (Fructose), the fructose diet containing 0.5% GTE (w/w) (Fructose + 0.5% GTE), or the fructose diet containing 1.0% GTE (Fructose + 1.0% GTE) for nine weeks (means ± SE; n = 8–9/group). Enzyme activities were measured spectrophotometrically in tissue homogenate. GLO-1 activity was determined by measuring the rate at which MGO-GSH hemithioacetal was converted to _S_-
d
-lactoylglutathione whereas GLO-2 activity was determined by measuring the disappearance of _S_-
d
/
l
-lactoylglutathione. Data were analyzed by 3-way ANOVA. Main effect of enzyme was significant (P < 0.001), but main effects of tissue and group were not (_P_ > 0.05). A significant tissue by enzyme interaction was observed (P < 0.001), but no other significant interactions were detected (_P_ > 0.05). *, significant difference between tissues for the same GLO enzyme (P < 0.01). $, significant difference between GLO enzymes within a tissue (P < 0.01).
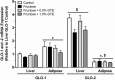
Figure 4
GLO-1 and GLO-2 mRNA expression in liver and adipose tissue. GLO-1 and GLO-2 mRNA expression are shown from rats fed a starch-based control diet containing 50% (w/w) starch and 10% (w/w) fructose (Control), a high-fructose diet containing 60% fructose (Fructose), the fructose diet containing 0.5% GTE (w/w) (Fructose + 0.5% GTE), or the fructose diet containing 1.0% GTE for nine weeks (Fructose + 1.0% GTE) (means ± SE; n = 8–9/group). RNA was isolated using TRIzol and reverse transcribed by MMLV reverse transcriptase for RT-PCR analysis using the primers described in Methods and Materials. Data were normalized to GLO-1 mRNA in the control group and analyzed by 3-way ANOVA. Main effects of tissue and enzyme as well as tissue by enzyme interaction were all significant (P < 0.01). There was no effect of diet (_P_ > 0.05) or interaction between diet and tissue (P > 0.05) or group (P > 0.05). A significant 3-way diet by tissue by enzyme interaction was observed (P < 0.05), but no pairwise differences between diets were detected (_P_ > 0.05). *, significant difference between tissues for the same GLO enzyme (P < 0.01). $, significant difference between GLO enzymes for within a tissue (P < 0.01).
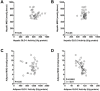
Figure 5
(a) Linear regression of MGO on hepatic GLO-1 activity. (b) Linear regression of hepatic MGO on hepatic GLO-2 activity. (c) Linear regression of adipose MGO on adipose GLO-1 activity. (d) Linear regression of adipose MGO on adipose GLO-2 activity. The relation between adipose GLO-2 and adipose MGO was significant (P < 0.0001), but the other relations were not (_P_ > 0.05).
Similar articles
- Acute glutathione depletion induces hepatic methylglyoxal accumulation by impairing its detoxification to D-lactate.
Masterjohn C, Mah E, Park Y, Pei R, Lee J, Manautou JE, Bruno RS. Masterjohn C, et al. Exp Biol Med (Maywood). 2013 Apr;238(4):360-9. doi: 10.1177/1535370213477987. Exp Biol Med (Maywood). 2013. PMID: 23760001 - Dietary Genistein Inhibits Methylglyoxal-Induced Advanced Glycation End Product Formation in Mice Fed a High-Fat Diet.
Zhao Y, Wang P, Sang S. Zhao Y, et al. J Nutr. 2019 May 1;149(5):776-787. doi: 10.1093/jn/nxz017. J Nutr. 2019. PMID: 31050753 - Dicarbonyl stress in clinical obesity.
Masania J, Malczewska-Malec M, Razny U, Goralska J, Zdzienicka A, Kiec-Wilk B, Gruca A, Stancel-Mozwillo J, Dembinska-Kiec A, Rabbani N, Thornalley PJ. Masania J, et al. Glycoconj J. 2016 Aug;33(4):581-9. doi: 10.1007/s10719-016-9692-0. Epub 2016 Jun 24. Glycoconj J. 2016. PMID: 27338619 Free PMC article. Review.
Cited by
- Mrr1 regulation of methylglyoxal catabolism and methylglyoxal-induced fluconazole resistance in Candida lusitaniae.
Biermann AR, Demers EG, Hogan DA. Biermann AR, et al. Mol Microbiol. 2021 Jan;115(1):116-130. doi: 10.1111/mmi.14604. Epub 2020 Dec 14. Mol Microbiol. 2021. PMID: 33319423 Free PMC article. - Attenuation of diabetic kidney injury in DPP4-deficient rats; role of GLP-1 on the suppression of AGE formation by inducing glyoxalase 1.
Sarker MK, Lee JH, Lee DH, Chun KH, Jun HS. Sarker MK, et al. Aging (Albany NY). 2020 Jan 6;12(1):593-610. doi: 10.18632/aging.102643. Epub 2020 Jan 6. Aging (Albany NY). 2020. PMID: 31905169 Free PMC article. - Effects of Acute Exposure to Methylglyoxal or/and A Diet Rich in Advanced Glycation End Products on Sperm Parameters in Mice.
Darmishonnejad Z, Hassan-Zadeh V, Tavalaee M, Kobarfard F, Gharagozloo P, Drevet JR, Nasr-Esfahani MH. Darmishonnejad Z, et al. Int J Fertil Steril. 2024 Jun 9;18(3):263-270. doi: 10.22074/ijfs.2023.2005832.1485. Int J Fertil Steril. 2024. PMID: 38973280 Free PMC article. - Transient Decrease in Circulatory Testosterone and Homocysteine Precedes the Development of Metabolic Syndrome Features in Fructose-Fed Sprague Dawley Rats.
Sakamuri A, Pitla S, Putcha UK, Jayapal S, Pothana S, Vadakattu SS, Konapalli NR, Sakamuri SS, Ibrahim A. Sakamuri A, et al. J Nutr Metab. 2016;2016:7510840. doi: 10.1155/2016/7510840. Epub 2016 Oct 12. J Nutr Metab. 2016. PMID: 27818793 Free PMC article. - Methylglyoxal-induced AMPK activation leads to autophagic degradation of thioredoxin 1 and glyoxalase 2 in HT22 nerve cells.
Dafre AL, Schmitz AE, Maher P. Dafre AL, et al. Free Radic Biol Med. 2017 Jul;108:270-279. doi: 10.1016/j.freeradbiomed.2017.03.028. Epub 2017 Mar 29. Free Radic Biol Med. 2017. PMID: 28363601 Free PMC article.
References
- McLellan A.C., Thornalley P.J., Benn J., Sonksen P.H. Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clin. Sci. (Lond.) 1994;87:21–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials