Self-propagation of pathogenic protein aggregates in neurodegenerative diseases - PubMed (original) (raw)
Review
Self-propagation of pathogenic protein aggregates in neurodegenerative diseases
Mathias Jucker et al. Nature. 2013.
Abstract
For several decades scientists have speculated that the key to understanding age-related neurodegenerative disorders may be found in the unusual biology of the prion diseases. Recently, owing largely to the advent of new disease models, this hypothesis has gained experimental momentum. In a remarkable variety of diseases, specific proteins have been found to misfold and aggregate into seeds that structurally corrupt like proteins, causing them to aggregate and form pathogenic assemblies ranging from small oligomers to large masses of amyloid. Proteinaceous seeds can therefore serve as self-propagating agents for the instigation and progression of disease. Alzheimer's disease and other cerebral proteopathies seem to arise from the de novo misfolding and sustained corruption of endogenous proteins, whereas prion diseases can also be infectious in origin. However, the outcome in all cases is the functional compromise of the nervous system, because the aggregated proteins gain a toxic function and/or lose their normal function. As a unifying pathogenic principle, the prion paradigm suggests broadly relevant therapeutic directions for a large class of currently intractable diseases.
Figures
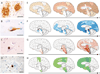
Figure 1. Commonalities among age-related neurodegenerative diseases
The deposited proteins adopt an amyloid conformation and show prion-like self-propagation and spreading in experimental settings, consistent with the progressive appearance of the lesions in the human diseases. a, Aβ deposits (senile plaques) in the neocortex of a patient with Alzheimer’s disease. b, Tau inclusion as a neurofibrillary tangle in a neocortical neuron of a patient with Alzheimer’s disease. c, α-Synuclein inclusion (Lewy body) in a neocortical neuron from a patient with Parkinson’s disease/Lewy body dementia. d, TDP-43 inclusion in a motoneuron of the spinal cord from a patient with amyotrophic lateral sclerosis. Scale bars are 50 µm in a and 20 µm in b–d. e–h, Characteristic progression of specific proteinaceous lesions in neurodegenerative diseases over time (t, black arrows), inferred from post-mortem analyses of brains. Aβ deposits and tau inclusions in brains of patients with Alzheimer’s disease (e and f), α-synuclein inclusions in brains of patients with Parkinson’s disease (g), and TDP-43 inclusions in brains of patients with amyotrophic lateral sclerosis (h). Three stages are shown for each disease, with white arrows indicating the putative spread of the lesions (for details see refs –8). Panels e and f are reproduced, with permission, from ref. .
Box 1. The amyloid state of proteins as a framework to explain prion-like protein seeding
A protein in the amyloid state forms bundles of twisted, unbranched filaments. Each filament is composed of sheets of β-strands (Panel a). These β-sheets run parallel to the filament axis, and the strands are nearly perpendicular to the long axis. This structural arrangement produces a distinctive, cross-β X-ray diffraction pattern that reflects the characteristic spacing between the β-sheets and the β-strands. Biophysicists classify amyloid based on the X-ray diffraction pattern, whereas pathologists define amyloid as deposits of fibrillar protein in cells or tissues that show reddish/green birefringence under cross-polarized light after staining with the dye Congo red. Part of panel a is modified, with permission, form ref. . In the most common amyloids, the β-sheets consist of parallel β-strands that are hydrogen-bonded by their backbones. The sheet is ‘in-register’ when identical side chains are on the top of each other. The sheets are bonded to each other via amino acid side chains that are inter-digitated like a zipper. Amyloid ‘steric zippers’ can be formed from identical or different β-strands (homosteric versus heterosteric zippers). An amyloid-forming protein may contribute more than one β-strand segment to the cross-β amyloid backbone (spine). At the molecular level, amyloids can be highly polymorphic; that is, a given β-strand segment is able to form a variety of distinct cross-β amyloid spines and filamentous structures (panel b). Such conformational variants are suggested to be the molecular basis of amyloid ‘strains’, that is, amyloids formed from a particular protein but with different biological activities,. Amyloid formation (panel c) starts with a slow nucleation phase (the aggregation of the protein into a seed) that may go through a series of intermediate states until the initial segment of the amyloid spine is formed,. Monomers or oligomeric structures are then bonded to the ends of the initial amyloid seed by conformational conversion. With increasing length, and depending on the conformational stability of the amyloid spine, the growing fibril can eventually break, either spontaneously or actively through cellular processes. In this way, amyloid formation becomes self-propagating through the generation and spread of new amyloid seeds. The kinetics of amyloid fibril formation are a function of the rates of nucleation, growth, and fragmentation,. The lag time that precedes protein aggregation in vitro can be greatly shortened by the addition of pre-formed exogenous seeds (panel d).
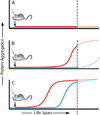
Box 2. Hypothetical model of seeded cerebral amyloid induction in mice
An endogenous protein (panel a, blue) is not amyloidogenic under physiological conditions. In this case, the application of a seed (panel a, red) will not induce protein aggregation. A protein is amyloidogenic but does not aggregate during the lifespan of the mouse (which ends at the dotted line), either because endogenously formed seeds are removed by an effective proteostasis network, or because seed formation is inefficient and therefore unlikely to occur during the mouse’s lifetime (panel b, blue). However, with an appropriate seed (single inoculation), the onset of protein aggregation is advanced and occurs before the mouse reaches the end of its lifespan (panel b, red). A protein is highly amyloidogenic and typically aggregates with ageing of the mouse (panel c, blue). The addition of an appropriate seed advances the onset of protein aggregation (panel c, red).
Similar articles
- Prions: generation and spread versus neurotoxicity.
Halliday M, Radford H, Mallucci GR. Halliday M, et al. J Biol Chem. 2014 Jul 18;289(29):19862-8. doi: 10.1074/jbc.R114.568477. Epub 2014 May 23. J Biol Chem. 2014. PMID: 24860100 Free PMC article. Review. - Proteopathic Strains and the Heterogeneity of Neurodegenerative Diseases.
Walker LC. Walker LC. Annu Rev Genet. 2016 Nov 23;50:329-346. doi: 10.1146/annurev-genet-120215-034943. Annu Rev Genet. 2016. PMID: 27893962 Free PMC article. Review. - Prion Diseases: A Unique Transmissible Agent or a Model for Neurodegenerative Diseases?
Ritchie DL, Barria MA. Ritchie DL, et al. Biomolecules. 2021 Feb 2;11(2):207. doi: 10.3390/biom11020207. Biomolecules. 2021. PMID: 33540845 Free PMC article. Review. - Amyloids, prions and the inherent infectious nature of misfolded protein aggregates.
Soto C, Estrada L, Castilla J. Soto C, et al. Trends Biochem Sci. 2006 Mar;31(3):150-5. doi: 10.1016/j.tibs.2006.01.002. Epub 2006 Feb 13. Trends Biochem Sci. 2006. PMID: 16473510 Review. - [The Propagation Hypothesis of Prion-like Protein Agregates in Neurodegenerative Diseases].
Nonaka T. Nonaka T. Brain Nerve. 2019 Nov;71(11):1209-1214. doi: 10.11477/mf.1416201430. Brain Nerve. 2019. PMID: 31722306 Japanese.
Cited by
- Integrating brainstem and cortical functional architectures.
Hansen JY, Cauzzo S, Singh K, García-Gomar MG, Shine JM, Bianciardi M, Misic B. Hansen JY, et al. Nat Neurosci. 2024 Oct 16. doi: 10.1038/s41593-024-01787-0. Online ahead of print. Nat Neurosci. 2024. PMID: 39414973 - Molecular Therapeutics in Development to Treat Alzheimer's Disease.
Tartaglia MC, Ingelsson M. Tartaglia MC, et al. Mol Diagn Ther. 2024 Sep 24. doi: 10.1007/s40291-024-00738-6. Online ahead of print. Mol Diagn Ther. 2024. PMID: 39316339 - α-Synuclein amyloid fibril directly binds to LC3B and suppresses SQSTM1/p62-mediated selective autophagy.
Xu Q, Wang H, Yang R, Tao Y, Wang Z, Zhang S, Sun B, Li D, Lu B, Liu C. Xu Q, et al. Cell Res. 2024 Sep 19. doi: 10.1038/s41422-024-01022-2. Online ahead of print. Cell Res. 2024. PMID: 39300253 No abstract available. - The prion principle and Alzheimer's disease.
Walker LC, Jucker M. Walker LC, et al. Science. 2024 Sep 20;385(6715):1278-1279. doi: 10.1126/science.adq5252. Epub 2024 Sep 19. Science. 2024. PMID: 39298592 - Latest Perspectives on Alzheimer's Disease Treatment: The Role of Blood-Brain Barrier and Antioxidant-Based Drug Delivery Systems.
Daraban BS, Popa AS, Stan MS. Daraban BS, et al. Molecules. 2024 Aug 27;29(17):4056. doi: 10.3390/molecules29174056. Molecules. 2024. PMID: 39274904 Free PMC article. Review.
References
- Malinovska L, Kroschwald S, Alberti S. Protein disorder, prion propensities, and self-organizing macromolecular collectives. Biochim. Biophys. Acta. 2013;1834:918–931. - PubMed
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. - PubMed
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. - PubMed
- Thal DR, Rub U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P51 OD011132/OD/NIH HHS/United States
- P51OD11132/OD/NIH HHS/United States
- P51 RR000165/RR/NCRR NIH HHS/United States
- R21 AG040589/AG/NIA NIH HHS/United States
- R21AG040589/AG/NIA NIH HHS/United States
- P51RR165/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials