PAF and EZH2 induce Wnt/β-catenin signaling hyperactivation - PubMed (original) (raw)
PAF and EZH2 induce Wnt/β-catenin signaling hyperactivation
Hae-Yun Jung et al. Mol Cell. 2013.
Abstract
Fine control of Wnt signaling is essential for various cellular and developmental decision-making processes. However, deregulation of Wnt signaling leads to pathological consequences, one of which is cancer. Here, we identify a function of PAF, a component of translesion DNA synthesis, in modulating Wnt signaling. PAF is specifically overexpressed in colon cancer cells and intestinal stem cells and is required for colon cancer cell proliferation. In Xenopus laevis, ventrovegetal expression of PAF hyperactivates Wnt signaling, developing a secondary axis with β-catenin target gene upregulation. Upon Wnt signaling activation, PAF dissociates from PCNA and binds directly to β-catenin. Then, PAF recruits EZH2 to the β-catenin transcriptional complex and specifically enhances Wnt target gene transactivation, independently of EZH2's methyltransferase activity. In mice, conditional expression of PAF induces intestinal neoplasia via Wnt signaling hyperactivation. Our studies reveal an unexpected role of PAF in regulating Wnt signaling and propose a regulatory mechanism of Wnt signaling during tumorigenesis.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
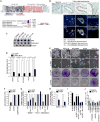
Figure 1. Mitogenic role of PAF in colon cancer cells
(A) Expression of PAF in human colon cancer cells. Oncomine analysis of PAF gene expression in human colon cancer cells. Comparison of Axin2 and PAF expression (GSE8671; fold change > 2;P value < 0.0001; gene rank < top 10 %) (upper panel). PAF expression in colon cancer tissues compared to normal tissues (fold change > 4; average P value = 9.34 × 10-6; gene rank < top 10%; N = sample number) (lower panel). (B) Upregulation of PAF in human colon cancer tissues. Human colon cancer tissue microarray samples were analyzed for immunohistochemistry (DAB [brown]: PAF; hematoxylin [blue]: nuclei) and immunofluorescent staining (arrowheads: nuclear PAF; e: epithelial cells; m: mesenchymal cells). Scale bar = 20 μm (C) Expression of PAF in human colon cancer cell lines. Colon cancer cell lines were fractionated into nuclear (N) and cytosolic (C) fractions for immunoblotting (IB). Fractionation controls: Histone H4 (nucleus) and tubulin (cytosol). (D and E) PAF Depletion inhibits cell proliferation. Each cell lines stably expressing shRNAs (sh-GFP [control] and sh-PAF) were analyzed using cell counting (D) and crystal violet staining (E); RU: relative units. (F) PCNA interaction-independent mitogenic role of PAF. SW620 (sh-GFP and sh-PAF) were stably transfected with nt-PAF or mutPIP-PAF for cell proliferation analysis (cell counting). (G) Downregulation of Cyclin D1 and c-Myc by PAF knockdown. SW620, Panc-1, and MDA-MB-231 cells stably expressing sh-GFP or sh-PAF were analyzed using quantitative reverse transcriptase PCR (qRT-PCR). (H) Axin2 downregulation by PAF depletion. SW620 and HCT116 cells were analyzed using qRT-PCR. (I) β-catenin rescues PAF depletion-induced cell growth inhibition. SW620 (sh-GFP and sh-PAF) cells were transfected with each plasmid, and analyzed by cell counting. N = 3. (J) β-catenin is not involved in activating MAPK and PI3K signalings. SW620 and HCT116 cells stably expressing β-catenin or empty vector were treated with LY294002 (PI3K inhibitor; 10 μm), U0216 (MEK1/2 inhibitor; 10 μm), or Sorafenib (Raf inhibitor; 10 μm). After 3 days, cells were counted. All error bars indicate standard deviation.
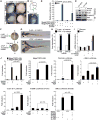
Figure 2. Activation of Wnt signaling by PAF
(A) PAF expression in AER. Whole-mount immunostaining for PAF in mouse embryos at embryonic day 11. Arrowheads: PAF. (B) Schematic diagram of AER in the limb bud. AER; dotted line; A, anterior; P, posterior; D, dorsal; V, ventral. (C) Active Wnt/β-catenin signaling in the AER. Axin2-LacZ mouse embryos at embryonic day 12 were stained with 5-bromo-4-chloro-indolyl-β-D-galactopyranoside (X-gal: arrowhead). (D and E) PAF knockdown suppresses β-catenin transcriptional activity. HeLa (sh-GFP or –PAF) were transiently transfected with β-catenin reporter and renilla plasmids. 24 h after transfection, cells were treated with 0.5 μM BIO. After 6 h, luciferase activity was measured (D). 293T cells stably transduced with sh-GFP or sh-PAF lentivirus were treated with Wnt3A (100 ng/ml, 6 h) for RT-PCR (E). (F-H) Axis duplication by xPAF-induced β-catenin hyperactivation. xPAF or β-galactosidase mRNA with β-catenin mRNA was injected into the ventrovegetal blastomeres of X. laevis embryos. The secondary axis was examined from neurulation (F) to tail bud stages (G). The x_β_-catenin mRNA concentration was titrated to prevent induction of axis duplication per se. A: anterior; P: posterior. (H) Two different doses of xPAF mRNA with β-catenin mRNA were injected into frog embryos for axis duplication analysis. (I) β-catenin target gene upregulation by xPAF. X. laevis embryos injected with each mRNA at two-cell stage were collected at the gastrulation stage for qRT-PCR. Ornithine decarboxylase (ODC): an internal control. (J) PAF-induced hyperactivation of Wnt/β-catenin target gene reporters. 293T cells transfected with each plasmid were treated with Wnt3A (100 ng/ml, 24 h) or LiCl (25 mM, 24 h), and analyzed for luciferase assays. N = 3. All error bars indicate standard deviation.
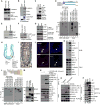
Figure 3. PAF-EZH2-β-catenin transcriptional complex at target gene promoters
(A) No effect of PAF on β-catenin protein stability. HeLa cells were transiently transfected with FLAG-PAF-pcDNA for IB. (B and C) Interaction of PAF with β-catenin and TCF/LEFs. GST-PAF was used for pull-down with SW620 cell lysates and IB (B). SW620 cells were analyzed for co-immunoprecipitation (co-IP) and IB (C). (D) PAF-β-catenin interaction via the armadillo repeat domain. GST-PAF was incubated with in vitro transcribed and translated FLAG-tagged β-catenin deletion mutants (N, N-term; Arm, Armadillo repeat domain; C, C-term) and analyzed using GST pull-down and IB. (E) PAF is a chromatin-associated protein. A chromatin-associated lysate (insoluble) and a soluble fraction of HCT116 were analyzed for IB. Brg-1: a chromatin fraction control. (F and G) PAF occupies TBEs. HCT116 stably expressing FLAG-PAF (F) and parental cells (G) were analyzed using ChIP. GAPDH promoter: a negative control. (H) Lgr5 positive and Bmi1 positive ISCs are located in the crypts. ISCs divide into transit-amplifying (TA) cells and differentiate into IECs. Wnt/β-catenin signaling is highly active in crypts containing ISCs and TA cells. (I) PAF expression in IECs of crypts. A small intestine tissue was immunostained for PAF (arrowhead); hematoxylin: blue. (J) PAF expression in Bmi1 positive ISCs. Immunofluorescent staining of murine colon tissue samples for PAF and Bmi1. Scale bars = 20 μm. (K) PAF interaction with EZH2. Co-IP and IB assays of SW620. H.C.; heavy chain. (L) PAF-EZH2 interaction via the CXC region. GST-PAF was incubated with HeLa cell lysates expressing each FLAG-EZH2 deletion mutant (A-D), and analyzed for GST pull-down and IB. EID, EED interaction domain; D1 and D2, homologous domains 1 and 2; CXC, cysteine-rich domain; SET, SU(var)3-9, E(z), and Trithorax histone methyltransferase domain. (M) Interaction of PAF with EZH2 and β-catenin, independently of PCNA. Co-IP of HCT116. A PCNA-immunodepleted (ID) supernatant was used for IP and IB. (N and O) Wnt-dependent PAF-β-catenin interaction. HeLa (vector or FLAG-PAF) were treated with LiCl (25 mM, 4 h) (N) or Wnt3A (100 ng/ml, 4 h) (O), and analyzed for co-IP and IB. L.E.: long exposure; S.E.: short exposure. (P) PAF-PCNA interaction in colon cancer cells. Co-IP and IB assays. Asterisks: IgG light chain.
Figure 4. PAF promotes EZH2-β-catenin interaction
(A) Constant association of EZH2, PAF, and β-catenin with the β-catenin target promoters. ChIP assays using HCT116 nuclear lysates. RNA Pol II: positive controls for the proximal promoter. (B) β-catenin-induced recruitment of EZH2 and PAF to promoters. HeLa cells (control and LiCl-treated; 25 mM, 4 h) were used for ChIP analysis. IgG and GAPDH served as negative controls for IP and ChIP-PCR, respectively. (C) ChIP promoter scanning of c-Myc promoter. HCT116 cells were analyzed by ChIP assays on the c-Myc promoter with 18 pairs of PCR primers. Average size of amplicons = 150 bp; green vertical bars: TBEs. (D) EZH2 association with β-catenin target promoters. EZH2 ChIP-seq data from mouse embryonic stem cells (GSE13084) were analyzed for EZH2 association in the promoters (200 kb) of β-catenin targets (Lef1, Lgr5, c-Myc, and Axin2). Ldha (lactate dehydrogenase A), Ubc (ubiquitin C), and Actb (β-actin): negative controls. (E) Downregulation of β-catenin targets by EZH2 depletion. HCT116 (sh-GFP or -EZH2) were analyzed by qRT-PCR. (F) EZH2 depletion inhibits PAF-mediated hyperactivation of β-catenin reporter activity. 293T cells (sh-GFP or -EZH2) were transfected with PAF and pMegaTOPFLASH plasmids, and treated with LiCl (25 mM, 24 h) for luciferase assays. (G) Recruitment of Pol II and Med1/TRAP220 to promoters upon β-catenin activation. ChIP assays using HeLa (control or LiCl [25 mM, 4 h]). (H) 293T cells transfected with EZH2, EZH2-F681I and pMegaTOPFLASH were analyzed for luciferase assays (N = 4). (I) ChIP assays of HCT116 sh-GFP or-PAF using semi-quantitative PCR. DCC promoter: a positive control for H3K27me3 (Derks et al., 2009). (J) PAF depletion impairs EZH2 recruitment to the c-Myc promoter. ChIP assays of HCT116 sh-GFP or –PAF using qPCR. Of note, PAF knockdown did not downregulate EZH2 (right IB panel). (K) β-catenin depletion decreases recruitment of PAF and EZH2 to promoters. ChIP assays of HCT116 (sh-GFP or sh-β-catenin). (L) PAF enhances EZH2-β-catenin interaction in vitro. GST-PAF pull-down of EZH2 and β-catenin protein mixture. EZH2-β-catenin interaction was then analyzed by co-IP and IB. (M) PAF increases β-catenin-EZH2 binding in vivo. HeLa (vector or PAF) treated with LiCl (25 mM, 4 h) analyzed for co-IP and IB. (N) Illustration of PAF-induced hyperactivation of the β-catenin transcriptional complex. In the setting of Wnt signaling activation, stabilized β-catenin sequesters PAF from PCNA. As a molecular adaptor, PAF facilitates interaction between β-catenin and EZH2. The EZH2/Mediator complex recruits RNA Pol II-associated transcriptional machinery to TBEs, and transactivates β-catenin target genes. All error bars indicate standard deviation.
Figure 5. Induction of intestinal neoplasia by PAF expression
(A) PAF conditional inducible mouse models. (B and C) Colonic crypts were isolated from Rosa26-rtTA:iPAF mice and maintained with or without doxy treatment (2 weeks). Phase-contrast images (B); quantification of the size of crypt organoids (C). (D-F) Induction of PAF expression develops intestinal microadenoma. Villin-Cre:Rosa26-LSL-rtTA:iPAF mice (experimental group) and iPAF mice (control) were given doxy (2 mg/ml in drinking water; small intestine [2 months] and colon [4 months]). Arrowheads: adenomas (D). H&E staining of small intestine (E) and colon (F). Dotted circles: aberrant crypt foci. (G) IEC hyperproliferation by PAF. Villin-Cre:Rosa26-LSL-rtTA:iPAF and iPAF mice were given doxy (2 months). Ki67 immunostaining; arrowheads: hyperplastic lesions. Scale bars = 50 μm. (H) Upregulation of CD44 by PAF. CD44 immunostaining of small intestine specimens from control and PAF-induced (4 months) mice. Scale bars = 500 μm. (I) PAF hyperactivates β-catenin reporter activity. X-gal staining of iPAF:BAT-gal and Rosa26-rtTA:iPAF:BAT-gal mice (doxy [7 days]). Dotted circles: endogenous Wnt signaling activity in crypts. (J) Upregulation of β-catenin target genes by PAF. Crypts isolated from Rosa26-rtTA:iPAF mice were treated with doxy (1 μg/ml, 36 h) for qRT-PCR. All error bars indicate standard deviation.
Comment in
- PAF makes it EZ(H2) for β-catenin transactivation.
Zhang X, He X. Zhang X, et al. Mol Cell. 2013 Oct 24;52(2):157-8. doi: 10.1016/j.molcel.2013.10.008. Mol Cell. 2013. PMID: 24210173 Free PMC article.
Similar articles
- PAF makes it EZ(H2) for β-catenin transactivation.
Zhang X, He X. Zhang X, et al. Mol Cell. 2013 Oct 24;52(2):157-8. doi: 10.1016/j.molcel.2013.10.008. Mol Cell. 2013. PMID: 24210173 Free PMC article. - Isoquercitrin suppresses colon cancer cell growth in vitro by targeting the Wnt/β-catenin signaling pathway.
Amado NG, Predes D, Fonseca BF, Cerqueira DM, Reis AH, Dudenhoeffer AC, Borges HL, Mendes FA, Abreu JG. Amado NG, et al. J Biol Chem. 2014 Dec 19;289(51):35456-67. doi: 10.1074/jbc.M114.621599. Epub 2014 Oct 30. J Biol Chem. 2014. PMID: 25359775 Free PMC article. - Wnt2 complements Wnt/β-catenin signaling in colorectal cancer.
Jung YS, Jun S, Lee SH, Sharma A, Park JI. Jung YS, et al. Oncotarget. 2015 Nov 10;6(35):37257-68. doi: 10.18632/oncotarget.6133. Oncotarget. 2015. PMID: 26484565 Free PMC article. - Genome-wide analysis of canonical Wnt target gene regulation in Xenopus tropicalis challenges β-catenin paradigm.
Nakamura Y, Hoppler S. Nakamura Y, et al. Genesis. 2017 Jan;55(1-2):e22991. doi: 10.1002/dvg.22991. Genesis. 2017. PMID: 28095618 Free PMC article. Review. - Wnt signaling in cell adhesion, development, and colon cancer.
Tejeda-Muñoz N, Mei KC. Tejeda-Muñoz N, et al. IUBMB Life. 2024 Jul;76(7):383-396. doi: 10.1002/iub.2806. Epub 2024 Jan 17. IUBMB Life. 2024. PMID: 38230869 Review.
Cited by
- Chromatin-Independent Interplay of NFATc1 and EZH2 in Pancreatic Cancer.
Patil S, Forster T, Reutlinger K, Kopp W, Versemann L, Spitalieri J, Gaedcke J, Ströbel P, Singh SK, Ellenrieder V, Neesse A, Hessmann E. Patil S, et al. Cells. 2021 Dec 8;10(12):3463. doi: 10.3390/cells10123463. Cells. 2021. PMID: 34943970 Free PMC article. - Malignant Peripheral Nerve Sheath Tumors: From Epigenome to Bedside.
Korfhage J, Lombard DB. Korfhage J, et al. Mol Cancer Res. 2019 Jul;17(7):1417-1428. doi: 10.1158/1541-7786.MCR-19-0147. Epub 2019 Apr 25. Mol Cancer Res. 2019. PMID: 31023785 Free PMC article. Review. - PAF promotes stemness and radioresistance of glioma stem cells.
Ong DST, Hu B, Ho YW, Sauvé CG, Bristow CA, Wang Q, Multani AS, Chen P, Nezi L, Jiang S, Gorman CE, Monasterio MM, Koul D, Marchesini M, Colla S, Jin EJ, Sulman EP, Spring DJ, Yung WA, Verhaak RGW, Chin L, Wang YA, DePinho RA. Ong DST, et al. Proc Natl Acad Sci U S A. 2017 Oct 24;114(43):E9086-E9095. doi: 10.1073/pnas.1708122114. Epub 2017 Oct 9. Proc Natl Acad Sci U S A. 2017. PMID: 29073105 Free PMC article. - Functional and therapeutic significance of EZH2 in urological cancers.
Liu X, Wu Q, Li L. Liu X, et al. Oncotarget. 2017 Jun 6;8(23):38044-38055. doi: 10.18632/oncotarget.16765. Oncotarget. 2017. PMID: 28410242 Free PMC article. Review. - Role of EZH2 in cancer stem cells: from biological insight to a therapeutic target.
Wen Y, Cai J, Hou Y, Huang Z, Wang Z. Wen Y, et al. Oncotarget. 2017 Jun 6;8(23):37974-37990. doi: 10.18632/oncotarget.16467. Oncotarget. 2017. PMID: 28415635 Free PMC article. Review.
References
- Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB., 3rd BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development. 2001;128:4449–4461. - PubMed
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. - PubMed
- Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous