Cas9 as a versatile tool for engineering biology - PubMed (original) (raw)
Review
Cas9 as a versatile tool for engineering biology
Prashant Mali et al. Nat Methods. 2013 Oct.
Abstract
RNA-guided Cas9 nucleases derived from clustered regularly interspaced short palindromic repeats (CRISPR)-Cas systems have dramatically transformed our ability to edit the genomes of diverse organisms. We believe tools and techniques based on Cas9, a single unifying factor capable of colocalizing RNA, DNA and protein, will grant unprecedented control over cellular organization, regulation and behavior. Here we describe the Cas9 targeting methodology, detail current and prospective engineering advances and suggest potential applications ranging from basic science to the clinic.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures
Figure 1
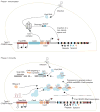
Functioning of the type II CRISPR-Cas systems in bacteria. Phase 1: in the immunization phase, the CRISPR system stores the molecular signature of a previous infection by integrating fragments of invading phage or plasmid DNA into the CRISPR locus as ‘spacers’. Phase 2: in the immunity phase, the bacterium uses this stored information to defend against invading pathogens by transcribing the locus and processing the resulting transcript to produce CRISPR RNAs (crRNAs) that guide effector nucleases to locate and cleave nucleic acids complementary to the spacer. First, tracrRNAs hybridize to repeat regions of the pre-crRNA. Second, endogenous RNase III cleaves the hybridized crRNA-tracrRNA, and a second event removes the 5′ end of the spacer, yielding mature crRNAs that remain associated with the tracrRNA and Cas9. The complex cleaves complementary ‘protospacer’ sequences only if a PAM sequence is present.
Figure 2
Cas9-sgRNA targeting complexes. (a) The basic S. pyogenes Cas9-sgRNA RNA-guided nuclease complex for eukaryotic genome engineering. Target recognition and cleavage require protospacer sequence complementary to the spacer and presence of the appropriate NGG PAM sequence at the 3′ of the protospacer. (b) Cas9 enables programmable localization of dsDNA, RNA and proteins. Proteins can be targeted to any dsDNA sequence by simply fusing them to Cas9nuclease-null, and additional RNA can be tethered to sgRNA termini without compromising Cas9 binding. Attaching RNA binding sites can in turn recruit RNA-binding proteins or directly recruit other RNAs via sequence hybridization. Finally, Cas9 can theoretically bring together any two dsDNA regions by employing sgRNA-Cas9 ‘staples’ that bind to the targeted loci and to one another. Consequently, Cas9 can in principle bring together any fusion proteins, any natural or fusion RNAs and/or any dsDNA locus to any other dsDNA sequence of interest. (c) The diverse potential applications of Cas9 range from targeted genome editing (via simplex and multiplex double-strand breaks and nicks) to targeted genome regulation (via tethering of epigenetic effector domains to either the Cas9 or sgRNA, and via competition with endogenous DNA binding factors) and possibly programmable genome reorganization and visualization. Cas9 might also be engineered to function as an RNA-guided recombinase, and via RNA tethers could serve as a scaffold for the assembly of multiprotein and nucleic acid complexes.
Figure 3
Platform for multiplex biological screens. To modulate multiple genomic sites, sgRNA libraries can be generated and delivered into target cells that also express orthogonal Cas9 effectors (nucleases, activators and/or repressors). This format enables multiplex ex vivo and in vivo genetic screens via targeted genome editing and/ or regulation.
Figure 4
Cas9 therapeutics. Potential Cas9-mediated therapeutic approaches include targeted genome editing to correct genetic disorders and targeted genome regulation to modify endogenous protein levels (top). Technical hurdles and potential routes to achieve these objectives are listed (bottom).
Similar articles
- RNA events. Cas9 targeting and the CRISPR revolution.
Barrangou R. Barrangou R. Science. 2014 May 16;344(6185):707-8. doi: 10.1126/science.1252964. Science. 2014. PMID: 24833384 No abstract available. - Selection and Validation of Spacer Sequences for CRISPR-Cas9 Genome Editing and Transcription Regulation in Bacteria.
Grenier F, Lucier JF, Rodrigue S. Grenier F, et al. Methods Mol Biol. 2015;1334:233-44. doi: 10.1007/978-1-4939-2877-4_15. Methods Mol Biol. 2015. PMID: 26404154 - Genome editing using Cas9 nickases.
Trevino AE, Zhang F. Trevino AE, et al. Methods Enzymol. 2014;546:161-74. doi: 10.1016/B978-0-12-801185-0.00008-8. Methods Enzymol. 2014. PMID: 25398340 - Genome editing. The new frontier of genome engineering with CRISPR-Cas9.
Doudna JA, Charpentier E. Doudna JA, et al. Science. 2014 Nov 28;346(6213):1258096. doi: 10.1126/science.1258096. Science. 2014. PMID: 25430774 Review. - Genome modification by CRISPR/Cas9.
Ma Y, Zhang L, Huang X. Ma Y, et al. FEBS J. 2014 Dec;281(23):5186-93. doi: 10.1111/febs.13110. Epub 2014 Nov 7. FEBS J. 2014. PMID: 25315507 Review.
Cited by
- Liver-targeted gene therapy: Approaches and challenges.
Aravalli RN, Belcher JD, Steer CJ. Aravalli RN, et al. Liver Transpl. 2015 Jun;21(6):718-37. doi: 10.1002/lt.24122. Liver Transpl. 2015. PMID: 25824605 Free PMC article. Review. - Advances in and Perspectives on Transgenic Technology and CRISPR-Cas9 Gene Editing in Broccoli.
Zhang L, Meng S, Liu Y, Han F, Xu T, Zhao Z, Li Z. Zhang L, et al. Genes (Basel). 2024 May 23;15(6):668. doi: 10.3390/genes15060668. Genes (Basel). 2024. PMID: 38927604 Free PMC article. Review. - Efficient generation of gene-modified pigs via injection of zygote with Cas9/sgRNA.
Wang Y, Du Y, Shen B, Zhou X, Li J, Liu Y, Wang J, Zhou J, Hu B, Kang N, Gao J, Yu L, Huang X, Wei H. Wang Y, et al. Sci Rep. 2015 Feb 5;5:8256. doi: 10.1038/srep08256. Sci Rep. 2015. PMID: 25653176 Free PMC article. - Systematic analysis of factors that improve homologous direct repair (HDR) efficiency in CRISPR/Cas9 technique.
Di Stazio M, Foschi N, Athanasakis E, Gasparini P, d'Adamo AP. Di Stazio M, et al. PLoS One. 2021 Mar 5;16(3):e0247603. doi: 10.1371/journal.pone.0247603. eCollection 2021. PLoS One. 2021. PMID: 33667229 Free PMC article. - Development of Cellular Models to Study Efficiency and Safety of Gene Edition by Homologous Directed Recombination Using the CRISPR/Cas9 System.
Sánchez-Hernández S, Aguilar-González A, Guijarro-Albaladejo B, Maldonado-Pérez N, Ramos-Hernández I, Cortijo-Gutiérrez M, Sánchez Martín RM, Benabdellah K, Martin F. Sánchez-Hernández S, et al. Cells. 2020 Jun 18;9(6):1492. doi: 10.3390/cells9061492. Cells. 2020. PMID: 32570971 Free PMC article.
References
- Bhaya D, Davison M, Barrangou R. CRISPR-Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annu Rev Genet. 2011;45:273–297. - PubMed
- Wiedenheft B, Sternberg SH, Doudna JA. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482:331–338. - PubMed
- Horvath P, Barrangou R. CRISPR/Cas, the immune system of bacteria and archaea. Science. 2010;327:167–170. - PubMed
- Cho SW, Kim S, Kim JM, Kim JS. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31:230–232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources