Aryl hydrocarbon receptor-dependent retention of nuclear HuR suppresses cigarette smoke-induced cyclooxygenase-2 expression independent of DNA-binding - PubMed (original) (raw)
Aryl hydrocarbon receptor-dependent retention of nuclear HuR suppresses cigarette smoke-induced cyclooxygenase-2 expression independent of DNA-binding
Michela Zago et al. PLoS One. 2013.
Abstract
The aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor that responds to man-made environmental toxicants, has emerged as an endogenous regulator of cyclooxygenase-2 (Cox-2) by a mechanism that is poorly understood. In this study, we first used AhR-deficient (AhR(-/-) ) primary pulmonary cells, together with pharmacological tools to inhibit new RNA synthesis, to show that the AhR is a prominent factor in the destabilization of Cox-2 mRNA. The destabilization of Cox-2 mRNA and subsequent suppression of cigarette smoke-induced COX-2 protein expression by the AhR was independent of its ability to bind the dioxin response element (DRE), thereby differentiating the DRE-driven toxicological AhR pathway from its anti-inflammatory abilities. We further describe that the AhR destabilizes Cox-2 mRNA by sequestering HuR within the nucleus. The role of HuR in AhR stabilization of Cox-2 mRNA was confirmed by knockdown of HuR, which resulted in rapid Cox-2 mRNA degradation. Finally, in the lungs of AhR(-/-) mice exposed to cigarette smoke, there was little Cox-2 mRNA despite robust COX-2 protein expression, a finding that correlates with almost exclusive cytoplasmic HuR within the lungs of AhR(-/-) mice. Therefore, we propose that the AhR plays an important role in suppressing the expression of inflammatory proteins, a function that extends beyond the ability of the AhR to respond to man-made toxicants. These findings open the possibility that a DRE-independent AhR pathway may be exploited therapeutically as an anti-inflammatory target.
Conflict of interest statement
Competing Interests: Both I, (Carolyn J. Baglole) and Dr. Imed Gallouzi are Editorial Board Members. This does not alter the authors’ adherence to all the PLOS ONE policies on sharing data and materials.
Figures
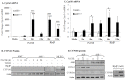
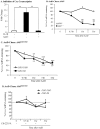
Figure 1. AhR activation by CSE does not increase COX-2 protein.
AhR−/− and AhR+/+ lung fibroblasts were exposed to CSE or B[_a_]P (1 µM) for 3, 6 or 24 hours and whole cell lysates collected for protein or RNA analysis. (A) There was a significant increase in Cyp1a1 mRNA in response to both CSE and B[_a_]P for 6 hours only in AhR+/+ cells (***p<0.0001). Results are expressed as the mean ± SEM of 3–6 independent experiments. (B) Basal levels of CYP1A1 protein were not detectable in primary lung fibroblasts. CYP1A1 was not increased by CSE or the AhR ligand TCDD. MLE-12 cells express basal CYP1A1 that was further increased by B[_a_]P treatment. Western blot is representative of three experiments. (C) There was significantly more Cyp1b1 mRNA in lung fibroblasts exposed to 1% CSE or B[_a_]P compared to AhR−/− cells. Results are expressed as the mean ± SEM of 3–8 independent experiments. (D) There is no CYP1B1 protein induction by CSE exposure for 24 hours; note the increase in COX-2 protein only in AhR−/− fibroblasts. B[_a_]P increased CYP1B1 protein expression in AhR+/+ fibroblasts. Representative western blot is shown.
Figure 2. Inhibition of AhR activity by the pharmacological antagonist CH-223191.
(A) Hepa.2Dluc cells were pre-treated with CH-223191 (10 µM) for one hour followed by treatment with B[a]P for 6 hours and cell lysates collected for luciferase activity. There was no induction in RLU when Hepa.2Dluc cells were treated with CH-223191 alone (ns = not significant compared to DMSO). There was a significant increase in RLU when Hepa.2Dluc were exposed to B[_a_]P (***p<0.0001 compared to DMSO). Pretreatment with CH-223191 dose-dependently inhibited luciferase activity elicited by B[_a_]P alone. Results are representative of two independent experiments and data are expressed as mean ± SEM. (B) There was a significant increase in CYP1B1 protein in mouse lung fibroblasts exposed to B[_a_]P; this increase was reduced by CH-223191. Results are expressed as the mean ± SEM of 3 independent experiments (*p<0.05); representative western blot is shown. (C) CSE-induced Cyp1a1 mRNA is significantly attenuated by CH-223191 in AhR-expressing mouse lung cells. Results are expressed as the mean ± SEM of 4 independent experiments (**p<0.01).
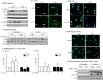
Figure 3. Inhibition of AhR activity augments CSE-induced COX-2 protein expression.
(A) There was a slight but detectible increase in COX-2 in the AhR+/− fibroblasts treated with CH-223191 alone (panel ii, arrowheads). When cells were exposed to both CSE and CH-223191, there was a strong induction of COX-2 (panel iv, arrows). Magnification = 40×. (B) There was a significant increase in the percentage of COX-2-positive cells in response to CH-223191 with or without CSE. Results are expressed as the mean ± SEM for 5 randomly-selected fields per triplicate experiment (**p<0.01). (C) There was a significant induction (fold-change: 4.93±2.4) in COX-2 protein expression when AhR-expressing cells were exposed to CH-223191 and 1% CSE compared to exposure to DMSO alone. Results are expressed as the mean ± SEM of 3 independent experiments. Representative western blot is shown. (D) Human lung fibroblasts- There was a slight but detectable increase in CSE-exposed human lung fibroblasts (panel iii). When cells were exposed to both 1% CSE and CH-223191, there was a stronger induction of COX-2 (panel iv, arrows). Magnification = 40×. (E) There was a significant increase in the percentage of COX-2-positive human lung fibroblasts in response to CH-223191 and exposed to CSE. Results are expressed as the mean ± SEM for 5 randomly-selected fields per triplicate experiment (**p<0.01; ***p<0.001). (F) There was a significant induction in COX-2 protein expression in human lung fibroblasts exposed to 1% CSE in conjunction with CH-223191 (3.1±0.84; *p<0.05 compared to both media control and 1% CSE alone). Representative western blot is shown. Results are expressed as the mean ± SEM of experiments utilizing fibroblasts from three different individuals.
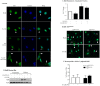
Figure 4. Suppression of CSE-induced COX-2 protein expression and PGE2 production does not require DNA binding activity of the AhR.
Lung fibroblasts were generated from AhRDBD/DBD or AhRDBD/B6 mice and treated with AhR ligands (TCDD, ITE and B[_a_]P) or 1% CSE and cellular RNA or protein was collected for qPCR/western blot analysis. Cell culture supernatant was assayed for PGE2 production. (A) AhRDBD/DBD and AhRDBD/B6 lung fibroblasts exposed to AhR ligands (TCDD, B[_a_]P or ITE) induce less CYP1B1 protein expression compared to lung fibroblasts from mice expressing one copy of the wild-type AhR. TCDD-exposed AhR+/+ and AhR−/− cells are included for comparison; note the lack of induction in the AhR−/− fibroblasts. (B) Cox-2 mRNA- There was a slight but not statistically significant increase in Cox-2 mRNA in AhRDBD/DBD cells (fold-change was 1.97±0.54; p = 0.5 compared to media-only). There was a significant increase in Cox-2 mRNA in AhRDBD/B6 fibroblasts in response to CSE (4.3±0.8; ***p<0.001 compared to media-only; p<0.01 compared to CSE-exposed AhRDBD/DBD cells). Results are expressed as mean ± SEM of 5 independent experiments. (C) There was a slight, but not significant, induction of COX-2 protein in response to 1% CSE. The relative level of induction in COX-2 protein was similar between AhRDBD/DBD and AhRDBD/B6 cells. Results are expressed as mean ± SEM of 5 independent experiments. Representative western blot is shown. (D) Baseline PGE2 levels did not differ between AhRDBD/DBD (235±73 pg/ml) and AhRDBD/B6 (247±99 pg/ml) cells. Exposure to 1% CSE did not significantly increase the concentration of PGE2 in either AhRDBD/DBD or AhRDBD/B6 lung fibroblasts. Samples were run in duplicate and the results are expressed as mean ± SEM of 3–6 independent experiments.
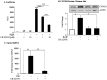
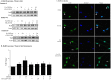
Figure 5. Transient expression of CSE-induced Cox-2 mRNA is due to AhR-dependent mRNA destabilization.
(A) In AhR+/− lung fibroblasts, there was a significant induction of Cox-2 mRNA by IL-1β (**p<0.05). Induction of _Cox-2_ mRNA was completely blocked by ActD (**p<0.05 compared to IL-1β-treated). Results are expressed as mean ± SEM of normalized _Cox-2_ levels and represent results from 3 independent experiments. (B) _AhR−/−_ and _AhR+/+_ lung fibroblasts were exposed to 1% CSE for 3 hours and then exposed to ActD (1 µg/ml) for the indicated time-points. _Cox-2_ levels were set to equal 100% after CSE exposure for three hours and are expressed as percentage (%) of _Cox-2_ mRNA remaining. _Cox-2_ mRNA expression remained relatively unchanged in _AhR−/−_ lung cells whereas there was a rapid and significant decline in _Cox-2_ mRNA in _AhR+/+_ cells after exposure to ActD (**p<0.01 compared to CSE-only exposed control cells; p<0.01 compared to AhR−/− lung at the respective time-point after ActD treatment). Results are expressed as mean ± SEM of normalized Cox-2 levels and represent data from 4 independent experiments. (C) AhRDBD/DBD and AhRDBD/B6 lung fibroblasts to 1% CSE for 3 hours followed by exposure to ActD (1 µg/ml) for the indicated time-points. There was a rapid and significant decline in Cox-2 mRNA in both AhRDBD/DBD and AhRDBD/B6 lung cells after exposure to ActD for 1, 3 or 6 hours (**p<0.01 and ***p<0.001 compared to CSE-only exposed control cells (time 0)). There was no significant difference in the percentage of Cox-2 mRNA levels remaining between AhRDBD/DBD and AhRDBD/B6 fibroblasts. Results are expressed as mean ± SEM of 2–6 independent experiments. (D) AhRDBD/DBD and AhRDBD/B6 lung fibroblasts were exposed to 1% CSE for 3 hours together with CH-223191 and ActD (1 µg/ml) for the indicated time-points. There was no significant decrease in the percentage of Cox-2 mRNA remaining when AhR activity is inhibited with CH-223191. Results are expressed as mean ± SEM of normalized Cox-2 levels and represent results from 3 independent experiments.
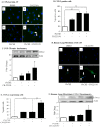
Figure 6. AhR retains HuR in the nucleus in response to CSE but does not contribute to HuR expression.
(A) HuR and CUGPB2 are constitutively expressed and are unaffected by AhR expression or CSE exposure. Note that there was an increase in Cox-2 protein in CSE-exposed AhR−/− fibroblasts but not AhR+/+ cells. (B) AhR−/− and AhR+/− lung fibroblasts were exposed to 1% CSE for 4 hours and IF performed for CUGBP2. Nuclei are visualized by Hoechst (blue) and the merged images are shown. CUGBP2 was localized predominantly in the nucleus in AhR−/− and AhR+/− fibroblasts (arrowheads), although cytoplasmic expression was detectable (arrows). Cytoplasmic CUGBP2 increased in both AhR−/− and AhR+/− fibroblasts exposed to 1% CSE (arrows). (C) In cells treated with media, HuR is predominantly localized in the nucleus both in AhR−/− and AhR+/− fibroblasts (arrowheads). CSE exposure (1%) for 4 hours in absence of AhR expression (AhR−/−) induces HuR shuttling from the nucleus to the cytoplasm (arrows). When AhR+/− fibroblasts are challenged with 1% CSE, HuR remains in the nucleus. Results are representative of three independent experiments. (D) There was an increase in cytoplasmic HuR only in the AhR−/− cells beginning at one hour of exposure and continuing through 4 hours. The purity of the extraction was determined by Tubulin, which was not detectable in the nuclear fraction. Representative western blot is shown. (E) Densitometric analysis of cytoplasmic and nuclear extracts following exposure to CSE: there was a significant increase in cytoplasmic HuR in response to CSE in only AhR−/− cells (2.5±0.3; *p<0.05 compared to media only; ¶p<0.05 compared to respective AhR+/− fibroblasts exposed to CSE at the indicated time-point). Results are expressed as mean ± SEM of three independent experiments. (F) Classic AhR ligands do not cause cellular HuR redistribution in mouse lung fibroblasts. HuR remained within the nucleus upon exposure to B[_a_]P. Images are representative of two independent experiments. Magnification = 40×. (G) There was no increase in cytoplasmic HuR in AhR+/+ or AhR−/− cells exposed to B[_a_]P for 4 hours. Representative western blot is shown.
Figure 7. Inhibition of AhR activity in mouse lung fibroblasts by CH-223191 promotes cytoplasmic shuttling of HuR in response to 1% CSE.
(A) AhR+/− mouse lung fibroblasts were pretreated with CH-223191 for one hour prior to being incubated with 1% CSE for an additional 4 hours and IF performed for HuR. HuR localization was restricted to the nucleus in media- and CSE exposed AhR+/− mouse lung fibroblasts (arrowheads). Cells that were treated with CH-223191 exhibited a slight increase in cytoplasmic expression of HuR (open arrows) while the majority of HuR remained within the nuclear compartment. When AhR+/− lung fibroblasts were pretreated with CH-223191, followed by incubation with 1% CSE, there was a pronounced increase in cytoplasmic HuR (closed arrows). Magnification = 40×; representative images are shown. (B) Western blot analysis of cytoplasmic and nuclear extracts was performed as described above following exposure of AhR+/+ lung fibroblasts to CSE with our without the AhR antagonist CH-223191 for 4 hours. There was an increase in cytoplasmic HuR in response to CH-223191 as well as CSE plus CH-223191. Representative western blot is shown. (C) There was a significant increase in the level of cytoplasmic HuR in response to CH-223191 (1.67±0.26) as well as 1% CSE plus CH-223191 (1.49±0.1). *p<0.05; results are expressed as mean ± SEM of 2 independent experiments. (D) HuR localization in response to CSE in AhRDBD/DBD fibroblasts. AhRDBD/DBD or control fibroblasts were exposed to 1% CSE for 4 hours and HuR localization assessed by IF as described above. HuR remained localized to the nucleus in both AhRDBD/DBD and AhRDBDB6 cells exposed to CSE. Magnification = 40×. (E) Percent-positive cells: There was no significant difference in the percentage of cells positive for cytoplasmic HuR between AhRDBD/DBD (16.6±7.5) and AhRDBD/B6 (20±2) cells after exposure to 1% CSE for 4 hours. The number of positive cells was also not different between media or exposure to CSE. Results are expressed as the mean ± SEM.
Figure 8. HuR expression and cellular localization in primary human lung fibroblasts after AhR inhibition by CH-223191.
(A) Primary human lung fibroblasts from three non-smoking individuals express HuR and the relative expression level was not altered by exposure to CSE. Inhibition of AhR activity with CH-223191 also had no affect on HuR protein levels. (B) Densitometric analysis indicated that there was a slight but not statistically significant change in HuR protein expression in response to CSE for 24 hours. Exposure to the AhR antagonist CH-223191, with or without CSE, also did not significantly alter HuR protein expression. Results are expressed as the mean ± SEM, n = 3 (western blots above). (C). HuR localization was restricted to the nucleus in human lung fibroblasts exposed to 1% CSE (arrowheads). Inhibition of AhR activity with CH-223191 slightly increased the amount of HuR in the cytoplasm (open arrows). The cytoplasmic redistribution of HuR was further augmented when cells were pretreated with CH-223191 and 1% CSE (open arrows). Images are representative of results obtained with lung fibroblasts derived from three different individuals.
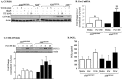
Figure 9. HuR silencing destabilizes Cox-2 mRNA in CSE-exposed AhR−/− lung fibroblasts.
Fibroblasts were transiently transfected with two siRNA against HuR (siRNA-1 and siRNA-2) or control (Ctrl) siRNA, exposed to CSE and Cox-2 mRNA stability evaluated by ActD chase experiments. (A) Transfection of AhR−/− fibroblasts with HuR siRNA significantly reduced HuR protein levels between 50–70%. Results are expressed as the mean ± SEM, n = 3 independent experiments per siRNA construct. (B) Cox-2 mRNA levels in AhR−/− fibroblasts transfected with Ctrl siRNA remained stable and did not significantly decline after exposure to ActD (ns = not significant compared to time 0). There was a significant decline in Cox-2 mRNA when HuR was knocked-down in AhR−/− cells (**p<0.01 compared to Time 0 of HuR siRNA). This decrease in Cox-2 mRNA following HuR siRNA-1 and siRNA-2 was significantly lower than the percentage of Cox-2 remaining in the Time 0 siRNA AhR−/− fibroblasts (*p<0.05; **p<0.01; ***p<0.001). Results are expressed as the mean ± SEM, n = 2–5 independent experiments. (C) AhR+/+ cells were transfected with two siRNA against HuR (siRNA-1 and siRNA-2); there was a significant reduction in HuR protein levels following knockdown (0.38±0.09- siRNA-1; 0.39±0.012- siRNA-2). Results are expressed as mean ± SEM of three independent experiments. (D) There was a significant decline in Cox-2 mRNA after exposure to ActD. Knock-down of HuR did not significantly affect the decay of Cox-2 mRNA levels (***p<0.001 compared to respective Time 0). There was no significant difference in the percentage of Cox-2 mRNA remaining between Ctrl, siRNA-1 or siRNA-2 (ns). Results are expressed as mean ± SEM of 2–4 independent experiments.
Figure 10. Cigarette smoke induction of pulmonary COX-2 protein expression in _AhR_-deficient mice is associated with increased cytoplasmic HuR.
(A) There was a significant induction of Cox-2 mRNA in the lungs of AhR+/− mice exposed to cigarette smoke for 2 (4.3±0.4) or 4 (4.4±1.0) weeks compared to air-exposed mice (***p<0.001). This induction in Cox-2 mRNA was significantly greater than CS-exposed AhR−/− mice ($$p<0.01). There was no induction of Cox-2 mRNA in the lungs of AhR−/− mice exposed to CS. Results are expressed as the mean ± SEM, n = 4–5 mice per group. (B) There is an increase in pulmonary COX-2 levels in response to CS in lung fibroblasts (orange/yellow color- arrows). Note that vimentin-negative cells (Hoechst- _blue color_- only) do not increase COX-2 in response to CS and likely reflect epithelial cells. Magnification = 40× and images are representative of COX-2 protein expression in the lungs of three different mice of each genotype. (C) COX-2 induction occurred in CS-exposed AhR−/− and AhR+/− mice. (D) Densitometric analysis revealed that CS elevates COX-2 in AhR−/− (2.2±0.4) and AhR+/− (2.5±0.39) mice. Results are expressed as the mean ± SEM, n = 7–8 mice per group. Representative western blot of 4–5 individual mice is shown. (E) The lungs of AhR+/− mice exposed to CS for 2 weeks exhibited some cytoplasmic translocation (open arrow) although HuR remained predominantly nuclear (pink color-arrowheads). There was a pronounced redistribution of HuR to the cytoplasm in the lungs of AhR−/− mice exposed to CS (open arrows). Inset shows enlarged region depicting difference in HuR localization; note the clearly visible nuclei (blue) in the AhR−/− lung. Images are representative of HuR in the lungs of three different mice of each genotype.
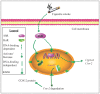
Figure 11. Schematic depiction of AhR-dependent attenuation of COX-2 protein by nuclear retention of HuR.
Cigarette smoke activates the AhR, which translocates to the nucleus and binds DNA, resulting in an increase in AhR-dependent gene transcription (e.g. Cyp1A1 and Cox-2 mRNA). The AhR also rapidly destabilizes Cox-2 mRNA by retaining HuR within the nucleus, suppressing an exaggerated increase in COX-2 protein expression. The AhR retention of nuclear HuR and subsequent suppression of COX-2 protein does not involve classic AhR:DNA binding but the mechanism by which AhR retains HuR within the nucleus is not known.
Similar articles
- Aryl hydrocarbon receptor (AhR)-dependent regulation of pulmonary miRNA by chronic cigarette smoke exposure.
Rogers S, de Souza AR, Zago M, Iu M, Guerrina N, Gomez A, Matthews J, Baglole CJ. Rogers S, et al. Sci Rep. 2017 Jan 12;7:40539. doi: 10.1038/srep40539. Sci Rep. 2017. PMID: 28079158 Free PMC article. - Low levels of the AhR in chronic obstructive pulmonary disease (COPD)-derived lung cells increases COX-2 protein by altering mRNA stability.
Zago M, Sheridan JA, Traboulsi H, Hecht E, Zhang Y, Guerrina N, Matthews J, Nair P, Eidelman DH, Hamid Q, Baglole CJ. Zago M, et al. PLoS One. 2017 Jul 27;12(7):e0180881. doi: 10.1371/journal.pone.0180881. eCollection 2017. PLoS One. 2017. PMID: 28749959 Free PMC article. - The aryl hydrocarbon receptor is a regulator of cigarette smoke induction of the cyclooxygenase and prostaglandin pathways in human lung fibroblasts.
Martey CA, Baglole CJ, Gasiewicz TA, Sime PJ, Phipps RP. Martey CA, et al. Am J Physiol Lung Cell Mol Physiol. 2005 Sep;289(3):L391-9. doi: 10.1152/ajplung.00062.2005. Epub 2005 Apr 29. Am J Physiol Lung Cell Mol Physiol. 2005. PMID: 15863442 - The aryl hydrocarbon receptor suppresses cigarette-smoke-induced oxidative stress in association with dioxin response element (DRE)-independent regulation of sulfiredoxin 1.
Sarill M, Zago M, Sheridan JA, Nair P, Matthews J, Gomez A, Roussel L, Rousseau S, Hamid Q, Eidelman DH, Baglole CJ. Sarill M, et al. Free Radic Biol Med. 2015 Dec;89:342-57. doi: 10.1016/j.freeradbiomed.2015.08.007. Epub 2015 Sep 25. Free Radic Biol Med. 2015. PMID: 26408075 - Molecular basis of tobacco smoke-induced premature skin aging.
Morita A, Torii K, Maeda A, Yamaguchi Y. Morita A, et al. J Investig Dermatol Symp Proc. 2009 Aug;14(1):53-5. doi: 10.1038/jidsymp.2009.13. J Investig Dermatol Symp Proc. 2009. PMID: 19675554 Review.
Cited by
- Aberrant Post-Transcriptional Regulation of Protein Expression in the Development of Chronic Obstructive Pulmonary Disease.
Aloufi N, Alluli A, Eidelman DH, Baglole CJ. Aloufi N, et al. Int J Mol Sci. 2021 Nov 4;22(21):11963. doi: 10.3390/ijms222111963. Int J Mol Sci. 2021. PMID: 34769392 Free PMC article. Review. - Aryl hydrocarbon receptor (AhR)-dependent regulation of pulmonary miRNA by chronic cigarette smoke exposure.
Rogers S, de Souza AR, Zago M, Iu M, Guerrina N, Gomez A, Matthews J, Baglole CJ. Rogers S, et al. Sci Rep. 2017 Jan 12;7:40539. doi: 10.1038/srep40539. Sci Rep. 2017. PMID: 28079158 Free PMC article. - Low levels of the AhR in chronic obstructive pulmonary disease (COPD)-derived lung cells increases COX-2 protein by altering mRNA stability.
Zago M, Sheridan JA, Traboulsi H, Hecht E, Zhang Y, Guerrina N, Matthews J, Nair P, Eidelman DH, Hamid Q, Baglole CJ. Zago M, et al. PLoS One. 2017 Jul 27;12(7):e0180881. doi: 10.1371/journal.pone.0180881. eCollection 2017. PLoS One. 2017. PMID: 28749959 Free PMC article. - Fibrotic microenvironment promotes the metastatic seeding of tumor cells via activating the fibronectin 1/secreted phosphoprotein 1-integrin signaling.
Zhang C, Wu M, Zhang L, Shang LR, Fang JH, Zhuang SM. Zhang C, et al. Oncotarget. 2016 Jul 19;7(29):45702-45714. doi: 10.18632/oncotarget.10157. Oncotarget. 2016. PMID: 27329720 Free PMC article. - Regional heterogeneity in response of airway epithelial cells to cigarette smoke.
Baskoro H, Sato T, Karasutani K, Suzuki Y, Mitsui A, Arano N, Nurwidya F, Kato M, Takahashi F, Kodama Y, Seyama K, Takahashi K. Baskoro H, et al. BMC Pulm Med. 2018 Sep 4;18(1):148. doi: 10.1186/s12890-018-0715-4. BMC Pulm Med. 2018. PMID: 30180847 Free PMC article.
References
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ (2006) Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 367: 1747–1757. - PubMed
- Barnes PJ (2000) Chronic obstructive pulmonary disease. N Engl J Med 343: 269–280. - PubMed
- Hayashi S, Watanabe J, Nakachi K, Eguchi H, Gotoh O, et al. (1994) Interindividual difference in expression of human Ah receptor and related P450 genes. Carcinogenesis 15: 801–806. - PubMed
- Baglole CJ, Maggirwar SB, Gasiewicz TA, Thatcher TH, Phipps RP, et al. (2008) The aryl hydrocarbon receptor attenuates tobacco smoke-induced cyclooxygenase-2 and prostaglandin production in lung fibroblasts through regulation of the NF-kappaB family member RelB. J Biol Chem 283: 28944–28957. - PMC - PubMed
- Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, et al. (2007) Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB. Am J Pathol 170: 855–864. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous