Heart fatty acid binding protein and Aβ-associated Alzheimer's neurodegeneration - PubMed (original) (raw)
Heart fatty acid binding protein and Aβ-associated Alzheimer's neurodegeneration
Rahul S Desikan et al. Mol Neurodegener. 2013.
Abstract
Background: Epidemiological and molecular findings suggest a relationship between Alzheimer's disease (AD) and dyslipidemia, although the nature of this association is not well understood.
Results: Using linear mixed effects models, we investigated the relationship between CSF levels of heart fatty acid binding protein (HFABP), a lipid binding protein involved with fatty acid metabolism and lipid transport, amyloid-β (Aβ), phospho-tau, and longitudinal MRI-based measures of brain atrophy among 295 non-demented and demented older individuals. Across all participants, we found a significant association of CSF HFABP with longitudinal atrophy of the entorhinal cortex and other AD-vulnerable neuroanatomic regions. However, we found that the relationship between CSF HABP and brain atrophy was significant only among those with low CSF Aβ1-42 and occurred irrespective of phospho-tau181p status.
Conclusions: Our findings indicate that Aβ-associated volume loss occurs in the presence of elevated HFABP irrespective of phospho-tau. This implicates a potentially important role for fatty acid binding proteins in Alzheimer's disease neurodegeneration.
Figures
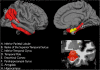
Figure 1
Three-dimensional representations of the neuroanatomic regions examined in the current study (only one hemisphere is shown). All of the examined neocortical regions are illustrated in the lateral and medial views of the gray matter surface (top row). The two non-neocortical regions (i.e., the hippocampus and amygdala) are illustrated in the coronal view of a T1-weighted MRI image (bottom row). Regions illustrated in red constitute the 'AD-vulnerable ROI’ (for further details please see manuscript text).
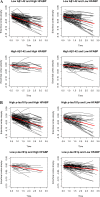
Figure 2
(A) Spaghetti plots illustrating atrophy of the entorhinal cortex among all participants classified as low Aβ 1–42 and high HFABP (based on median value of FABP) (top left panel), low Aβ 1–42 and low HFABP (top right panel), high Aβ 1–42 and high FABP (bottom left panel), and high Aβ 1–42 and low FABP (bottom right panel). The red line indicates the mean atrophy rate for the four respective groups (i.e. low Aβ1–42 and high FABP, low Aβ1–42 and low FABP, high Aβ1–42 and high FABP and high Aβ1–42 and low FABP). As illustrated, the slopes of the red lines are significantly different depending on CSF Aβ1–42 status (please see text for further details). (B) Spaghetti plots illustrating atrophy of the entorhinal cortex among all participants classified as high p-tau181p and high HFABP (based on median value of FABP) (top left panel), high p-tau181p and low HFABP (top right panel), low p-tau181p and high FABP (bottom left panel), and low p-tau181p and low FABP (bottom right panel). The red line indicates the mean atrophy rate for the four respective groups (i.e. high p-tau181p and high FABP, high p-tau181p and low FABP, low p-tau181p and high FABP and low p-tau181p and low FABP). As illustrated, the slopes of the red lines are not significantly different depending on CSF p-tau181p status (please see text for further details).
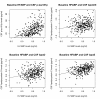
Figure 3
Scatter plots demonstrating the relationship between baseline CSF levels of HFABP (quality-controlled, transformed values as described in reference 18) CSF p-tau 181p (top left), CSF ApoC III (top right), CSF ApoD (bottom left) and CSF ApoE (bottom right). The black line represents the best-fit regression line.
Similar articles
- The role of clusterin in amyloid-β-associated neurodegeneration.
Desikan RS, Thompson WK, Holland D, Hess CP, Brewer JB, Zetterberg H, Blennow K, Andreassen OA, McEvoy LK, Hyman BT, Dale AM; Alzheimer’s Disease Neuroimaging Initiative Group. Desikan RS, et al. JAMA Neurol. 2014 Feb;71(2):180-7. doi: 10.1001/jamaneurol.2013.4560. JAMA Neurol. 2014. PMID: 24378367 Free PMC article. - CSF levels of heart fatty acid binding protein are altered during early phases of Alzheimer's disease.
Chiasserini D, Parnetti L, Andreasson U, Zetterberg H, Giannandrea D, Calabresi P, Blennow K. Chiasserini D, et al. J Alzheimers Dis. 2010;22(4):1281-8. doi: 10.3233/JAD-2010-101293. J Alzheimers Dis. 2010. PMID: 20930282 - Heart-type fatty acid binding protein and vascular endothelial growth factor: cerebrospinal fluid biomarker candidates for Alzheimer's disease.
Guo LH, Alexopoulos P, Perneczky R. Guo LH, et al. Eur Arch Psychiatry Clin Neurosci. 2013 Oct;263(7):553-60. doi: 10.1007/s00406-013-0405-4. Epub 2013 Apr 17. Eur Arch Psychiatry Clin Neurosci. 2013. PMID: 23591828 - Clinical indications for analysis of Alzheimer's disease CSF biomarkers.
Engelborghs S. Engelborghs S. Rev Neurol (Paris). 2013 Oct;169(10):709-14. doi: 10.1016/j.neurol.2013.07.024. Epub 2013 Sep 6. Rev Neurol (Paris). 2013. PMID: 24016466 Review.
Cited by
- The relationship between complement factor C3, APOE ε4, amyloid and tau in Alzheimer's disease.
Bonham LW, Desikan RS, Yokoyama JS; Alzheimer’s Disease Neuroimaging Initiative. Bonham LW, et al. Acta Neuropathol Commun. 2016 Jun 29;4(1):65. doi: 10.1186/s40478-016-0339-y. Acta Neuropathol Commun. 2016. PMID: 27357286 Free PMC article. - Early role of vascular dysregulation on late-onset Alzheimer's disease based on multifactorial data-driven analysis.
Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC; Alzheimer’s Disease Neuroimaging Initiative. Iturria-Medina Y, et al. Nat Commun. 2016 Jun 21;7:11934. doi: 10.1038/ncomms11934. Nat Commun. 2016. PMID: 27327500 Free PMC article. - Intrathecal 2-hydroxypropyl-β-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1-2 trial.
Ory DS, Ottinger EA, Farhat NY, King KA, Jiang X, Weissfeld L, Berry-Kravis E, Davidson CD, Bianconi S, Keener LA, Rao R, Soldatos A, Sidhu R, Walters KA, Xu X, Thurm A, Solomon B, Pavan WJ, Machielse BN, Kao M, Silber SA, McKew JC, Brewer CC, Vite CH, Walkley SU, Austin CP, Porter FD. Ory DS, et al. Lancet. 2017 Oct 14;390(10104):1758-1768. doi: 10.1016/S0140-6736(17)31465-4. Epub 2017 Aug 10. Lancet. 2017. PMID: 28803710 Free PMC article. Clinical Trial. - Exercise to Counteract Alzheimer's Disease: What Do Fluid Biomarkers Say?
Bonanni R, Cariati I, Cifelli P, Frank C, Annino G, Tancredi V, D'Arcangelo G. Bonanni R, et al. Int J Mol Sci. 2024 Jun 25;25(13):6951. doi: 10.3390/ijms25136951. Int J Mol Sci. 2024. PMID: 39000060 Free PMC article. Review. - Reasons for Failed Trials of Disease-Modifying Treatments for Alzheimer Disease and Their Contribution in Recent Research.
Yiannopoulou KG, Anastasiou AI, Zachariou V, Pelidou SH. Yiannopoulou KG, et al. Biomedicines. 2019 Dec 9;7(4):97. doi: 10.3390/biomedicines7040097. Biomedicines. 2019. PMID: 31835422 Free PMC article. Review.
References
- Jones L, Holmans PA, Hamshere ML, Harold D, Moskvina V, Ivanov D, Pocklington A, Abraham R, Hollingworth P, Sims R, Gerrish A, Pahwa JS, Jones N, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schürmann B, Heun R, Kölsch H, van den Bussche H, Heuser I, Peters O, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Hüll M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel KH, Klopp N, Wichmann HE, Rüther E, Carrasquillo MM, Pankratz VS, Younkin SG, Hardy J, O’Donovan MC, Owen MJ, Williams J. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS One. 2010;5:e13950. doi: 10.1371/journal.pone.0013950. - DOI - PMC - PubMed
- Jones L, Harold D, Williams J. Genetic evidence for the involvement of lipid metabolism in Alzheimer’s disease. Biochim Biophys Acta. 1801;2010:754–761. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG005131/AG/NIA NIH HHS/United States
- K01AG029218/AG/NIA NIH HHS/United States
- K02 NS067427/NS/NINDS NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- K01 AG030514/AG/NIA NIH HHS/United States
- AG010129/AG/NIA NIH HHS/United States
- R01AG031224/AG/NIA NIH HHS/United States
- T32 EB005970/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous