First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics - PubMed (original) (raw)
Review
First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics
Hazel H Szeto. Br J Pharmacol. 2014 Apr.
Abstract
A decline in energy is common in aging, and the restoration of mitochondrial bioenergetics may offer a common approach for the treatment of numerous age-associated diseases. Cardiolipin is a unique phospholipid that is exclusively expressed on the inner mitochondrial membrane where it plays an important structural role in cristae formation and the organization of the respiratory complexes into supercomplexes for optimal oxidative phosphorylation. The interaction between cardiolipin and cytochrome c determines whether cytochrome c acts as an electron carrier or peroxidase. Cardiolipin peroxidation and depletion have been reported in a variety of pathological conditions associated with energy deficiency, and cardiolipin has been identified as a target for drug development. This review focuses on the discovery and development of the first cardiolipin-protective compound as a therapeutic agent. SS-31 is a member of the Szeto-Schiller (SS) peptides known to selectively target the inner mitochondrial membrane. SS-31 binds selectively to cardiolipin via electrostatic and hydrophobic interactions. By interacting with cardiolipin, SS-31 prevents cardiolipin from converting cytochrome c into a peroxidase while protecting its electron carrying function. As a result, SS-31 protects the structure of mitochondrial cristae and promotes oxidative phosphorylation. SS-31 represents a new class of compounds that can recharge the cellular powerhouse and restore bioenergetics. Extensive animal studies have shown that targeting such a fundamental mechanism can benefit highly complex diseases that share a common pathogenesis of bioenergetics failure. This review summarizes the mechanisms of action and therapeutic potential of SS-31 and provides an update of its clinical development programme.
Keywords: SS-31; Szeto-Schiller peptides; bendavia; cytochrome c; cytochrome c peroxidase; mitochondria cristae; mitochondrial permeability transition; oxidative stress; reactive oxygen species.
© 2013 The British Pharmacological Society.
Figures
Figure 1
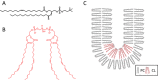
Cardiolipin promotes curvature in lipid membranes due to its unique conical structure. (A) Chemical structure of phosphatidylcholine (PC). (B) Chemical structure of cardiolipin (CL). (C) Cardiolipin exerts lateral pressure in a lipid bilayer to induce a negative curvature.
Figure 2
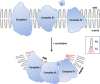
Cardiolipin is important for cristae structure and supercomplex formation on the inner mitochondrial membrane (IMM). The protein complexes of the electron transport chain reside on cristae membranes and cardiolipin (CL) provides curvatures on the IMM to increase surface area for the respiratory complexes. Cardiolipin also helps to organize the respiratory complexes into supercomplexes to facilitate electron transfer among the redox partners. Lastly, cardiolipin anchors the highly cationic cyt c (C) via electrostatic interaction to bring it in close proximity to Complex III and Complex IV for efficient electron transfer. IMS: intermembrane space.
Figure 3
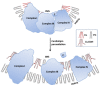
Cardiolipin peroxidation destabilizes cardiolipin microdomains on the inner mitochondrial membrane (IMM) and disrupts supercomplexes. Cardiolipin (CL) is particularly vulnerable to oxidative damage because of its high content of unsaturated fatty acids. Peroxidation of the acyl chains alters the structure of cardiolipin (CLOOH) and prevents cardiolipin from aggregating into microdomains or rafts on the IMM. The breakdown of cardiolipin rafts abolishes cristae curvatures and disrupts the organization of respiratory complexes into higher order supercomplexes. Peroxidation of cardiolipin also reduces its affinity for cyt c (C) and sets the stage for cyt c release into the cytosol and apoptosis. IMS: intermembrane space.
Figure 4
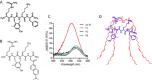
SS-31 selectively binds to cardiolipin. (A) Chemical structure of SS-31 (D-Arg-dimethylTyr-Lys-Phe-NH2). (B) Chemical structure of [ald]SS-31. The Phe4 in SS-31 is replaced by aladan (ald), a polarity-sensitive fluorescent amino acid. (C) Fluorescence emission spectra of [ald]SS-31 in the absence of phospholipids (no PL), or in the presence of cardiolipin (CL), phosphatidylcholine (PC) or phosphatidylethanolamine (PE). Addition of cardiolipin caused a shift of the emission maximum (λmax) from 530 to 500 nm. (D) Proposed model showing the interaction of SS-31 with cardiolipin. Electrostatic interaction between the two cationic moieties of SS-31 (Arg and Lys) and the phosphate head groups of cardiolipin aligns the aromatic residues (dimethyl-Tyr and Phe) within the hydrophobic acyl chain region of cardiolipin.
Figure 5
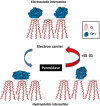
Interaction of SS-31 with cardiolipin favours the electron carrier over the peroxidase in cyt c. Electrostatic interaction between cyt c and cardiolipin brings cyt c in close proximity to the respiratory complexes for optimal electron transfer. However, hydrophobic interaction between cyt c and cardiolipin results in unfolding of the tertiary structure of cyt c and converts this electron carrier into a peroxidase by disrupting the Met80-haem ligation. The SS-31/cardiolipin complex localizes to within angstroms of the haem group to protect the Met80-haem coordination and inhibits peroxidase activity while improving π−π* interaction to promote electron transfer and ATP synthesis.
Figure 6
SS-31 protects mitochondrial cristae during ischaemia and prevents mitochondrial permeability transition. Rats were treated with saline or SS-31 (2 mg kg−1) before occlusion of renal blood flow for 45 min. Kidney sections were obtained either at the end of ischaemia or after 5 min reperfusion and examined by transmission electron microscopy. (A) Representative electron microscopic image of proximal tubular cells from saline-treated animal at the end of ischaemia shows swollen mitochondria with loss of cristae and matrix material (*). (B) Mitochondria from SS-31 treated kidneys looked normal and were elongated with finely stacked cristae membranes even after 45 min ischaemia. (C) Mitochondria from saline-treated animals remained swollen (*) after 5 min reperfusion and some of them showed breaks in the outer mitochondrial membrane and loss of matrix contents into the cytosol (arrow), consistent with mitochondrial permeability transition. (D) Mitochondria from SS-31 treated sections were normal with many cristae stacks. All images are taken at 80 000 × magnification.
Similar articles
- Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis.
Birk AV, Chao WM, Bracken C, Warren JD, Szeto HH. Birk AV, et al. Br J Pharmacol. 2014 Apr;171(8):2017-28. doi: 10.1111/bph.12468. Br J Pharmacol. 2014. PMID: 24134698 Free PMC article. - The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin.
Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH. Birk AV, et al. J Am Soc Nephrol. 2013 Jul;24(8):1250-61. doi: 10.1681/ASN.2012121216. Epub 2013 Jul 11. J Am Soc Nephrol. 2013. PMID: 23813215 Free PMC article. - Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis.
Liu S, Soong Y, Seshan SV, Szeto HH. Liu S, et al. Am J Physiol Renal Physiol. 2014 May 1;306(9):F970-80. doi: 10.1152/ajprenal.00697.2013. Epub 2014 Feb 19. Am J Physiol Renal Physiol. 2014. PMID: 24553434 - Cardiolipin-targeted peptides rejuvenate mitochondrial function, remodel mitochondria, and promote tissue regeneration during aging.
Szeto HH, Liu S. Szeto HH, et al. Arch Biochem Biophys. 2018 Dec 15;660:137-148. doi: 10.1016/j.abb.2018.10.013. Epub 2018 Oct 23. Arch Biochem Biophys. 2018. PMID: 30359579 Review. - Serendipity and the discovery of novel compounds that restore mitochondrial plasticity.
Szeto HH, Birk AV. Szeto HH, et al. Clin Pharmacol Ther. 2014 Dec;96(6):672-83. doi: 10.1038/clpt.2014.174. Epub 2014 Sep 4. Clin Pharmacol Ther. 2014. PMID: 25188726 Free PMC article. Review.
Cited by
- Mitochondrial dysfunction in cardiac aging.
Tocchi A, Quarles EK, Basisty N, Gitari L, Rabinovitch PS. Tocchi A, et al. Biochim Biophys Acta. 2015 Nov;1847(11):1424-33. doi: 10.1016/j.bbabio.2015.07.009. Epub 2015 Jul 17. Biochim Biophys Acta. 2015. PMID: 26191650 Free PMC article. Review. - Reduced adenosine diphosphate sensitivity in skeletal muscle mitochondria increases reactive oxygen species production in mouse models of aging and oxidative stress but not denervation.
Pharaoh G, Brown J, Ranjit R, Ungvari Z, Van Remmen H. Pharaoh G, et al. JCSM Rapid Commun. 2021 Jan-Jun;4(1):75-89. doi: 10.1002/rco2.29. Epub 2020 Dec 28. JCSM Rapid Commun. 2021. PMID: 36159599 Free PMC article. - Beneficial effects of SS-31 peptide on cardiac mitochondrial dysfunction in tafazzin knockdown mice.
Russo S, De Rasmo D, Signorile A, Corcelli A, Lobasso S. Russo S, et al. Sci Rep. 2022 Nov 18;12(1):19847. doi: 10.1038/s41598-022-24231-4. Sci Rep. 2022. PMID: 36400945 Free PMC article. - Role of mitochondria in endogenous renal repair.
Kazeminia S, Eirin A. Kazeminia S, et al. Clin Sci (Lond). 2024 Aug 7;138(15):963-973. doi: 10.1042/CS20231331. Clin Sci (Lond). 2024. PMID: 39076039 Free PMC article. Review. - The Vicious Cycle of Renal Lipotoxicity and Mitochondrial Dysfunction.
Ge M, Fontanesi F, Merscher S, Fornoni A. Ge M, et al. Front Physiol. 2020 Jul 7;11:732. doi: 10.3389/fphys.2020.00732. eCollection 2020. Front Physiol. 2020. PMID: 32733268 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials