LRP1 assembles unique co-receptor systems to initiate cell signaling in response to tissue-type plasminogen activator and myelin-associated glycoprotein - PubMed (original) (raw)
LRP1 assembles unique co-receptor systems to initiate cell signaling in response to tissue-type plasminogen activator and myelin-associated glycoprotein
Elisabetta Mantuano et al. J Biol Chem. 2013.
Abstract
In addition to functioning as an activator of fibrinolysis, tissue-type plasminogen activator (tPA) interacts with neurons and regulates multiple aspects of neuronal cell physiology. In this study, we examined the mechanism by which tPA initiates cell signaling in PC12 and N2a neuron-like cells. We demonstrate that enzymatically active and inactive tPA (EI-tPA) activate ERK1/2 in a biphasic manner. Rapid ERK1/2 activation is dependent on LDL receptor-related protein-1 (LRP1). In the second phase, ERK1/2 is activated by tPA independently of LRP1. The length of the LRP1-dependent phase varied inversely with the tPA concentration. Rapid ERK1/2 activation in response to EI-tPA and activated α2-macroglobulin (α2M*) required the NMDA receptor and Trk receptors, which assemble with LRP1 into a single pathway. Assembly of this signaling system may have been facilitated by the bifunctional adapter protein, PSD-95, which associated with LRP1 selectively in cells treated with EI-tPA or α2M*. Myelin-associated glycoprotein binds to LRP1 with high affinity but failed to induce phosphorylation of TrkA or ERK1/2. Instead, myelin-associated glycoprotein recruited p75 neurotrophin receptor (p75NTR) into a complex with LRP1 and activated RhoA. p75NTR was not recruited by other LRP1 ligands, including EI-tPA and α2M*. Lactoferrin functioned as an LRP1 signaling antagonist, inhibiting Trk receptor phosphorylation and ERK1/2 activation in response to EI-tPA. These results demonstrate that LRP1-initiated cell signaling is ligand-dependent. Proteins that activate cell signaling by binding to LRP1 assemble different co-receptor systems. Ligand-specific co-receptor recruitment provides a mechanism by which one receptor, LRP1, may trigger different signaling responses.
Keywords: Cell Signaling; ERK; Lipoprotein-like Receptor (LRP); Protease Inhibitor; Tissue-type Plasminogen Activator (tPA).
Figures
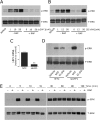
FIGURE 1.
The role of LRP1 in ERK1/2 activation by EI-tPA and α2M*. PC12 cells were treated with vehicle (SFM) or with increasing concentrations of α2M* (1–50 n
m
) (A) or EI-tPA (1–50 n
m
) (B) for 10 min. GST-RAP (250 n
m
) was added to the cultures 30 min before α2M* or EI-tPA, as indicated (+RAP). C, PC12 cells were transfected with NTC or _LRP1_-specific siRNA. Relative LRP1 mRNA expression was determined by qPCR. D, transfected PC12 cells were treated with NGF-β (50 ng/ml) or EI-tPA (12 n
m
) for 10 min. E, PC12 cells were pretreated with GST-RAP (250 n
m
) for 30 min (+RAP) or with vehicle (−RAP) and then with EI-tPA (12 n
m
) for the indicated times, up to 2 h. Equal amounts of cellular protein (50 μg) were loaded into each lane and subjected to SDS-PAGE. Immunoblot analysis was performed to detect phosphorylated ERK1/2 (p-ERK) and total ERK (T-ERK). The blots shown are representative of at least three independent studies. **, p ≤ 0.005.
FIGURE 2.
Enzymatically active tPA and EI tPA demonstrate similar activity. A, PC12 cells were pretreated with 250 n
m
GST-RAP (+RAP) or with vehicle (−RAP) for 30 min and then with increasing concentrations of EI-tPA (2–60 n
m
) for 10 min. B, PC12 cells were pretreated with 250 n
m
GST-RAP (+RAP) or with vehicle (−RAP) for 30 min and then treated with EI-tPA (12 n
m
) for 10 min or with enzymatically active tPA (12 n
m
) for different periods of times up to 1 h. Immunoblot analysis was performed to detect phosphorylated ERK1/2 (p-ERK) and total ERK1/2 (T-ERK).
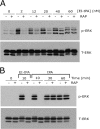
FIGURE 3.
EI-tPA-initiated cell signaling is biphasic in relation to the role of LRP1 in N2a cells. A, N2a cells were pretreated with RAP (250 n
m
) for 30 min as indicated (+RAP) and then treated with vehicle (SFM), α2M* (10 n
m
), or EI-tPA (12 n
m
) for 10 min. B, N2a cells were pretreated with GST-RAP (250 n
m
) for 30 min, as indicated (+RAP), and then treated with EI-tPA (12 n
m
) for different times up to 2 h. C, N2a cells were pretreated with GST-RAP (250 n
m
) for 30 min and then with increasing concentrations of EI-tPA (2–60 n
m
). Immunoblot analysis was performed to detect phosphorylated ERK1/2 (p-ERK) and total ERK1/2 (T-ERK).
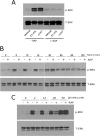
FIGURE 4.
NMDA-R is required for ERK1/2 activation by EI-tPA and α2M*. A, PC12 cells were pretreated with MK-801 (1 μ
m
) or vehicle for 2 h and then treated with vehicle (SFM), NGF-β (50 ng/ml), or EI-tPA (12 n
m
) for 10 min. B, N2a cells were pretreated with MK-801 (1 μ
m
) for 2 h and then treated with vehicle (SFM), EI-tPA (12 n
m
), or α2M* (10 n
m
) for 10 min. C, PC12 cells were transfected with NTC siRNA or _NR1_-specific siRNA (siNR1). NR1 mRNA was determined by qPCR. NR1 protein was determined by immunoblot analysis 48 h after transfection. D, PC12 cells in which NR1 was silenced and control cells transfected with NTC siRNA were treated with NGF-β (50 ng/ml) or EI-tPA (12 n
m
) for 10 min. E, PC12 cells in which NR1 was silenced and control cells transfected with NTC siRNA were treated with EI-tPA (12 n
m
) for different times up to 2 h. Immunoblot analysis was performed to detect phosphorylated ERK1/2 (p-ERK) and total ERK1/2 (T-ERK). *, p < 0.05.
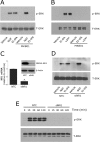
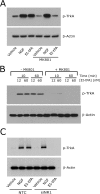
FIGURE 5.
NMDA-R is required for Trk receptor transactivation. A, PC12 cells were pretreated with MK-801 (1 μ
m
) for 2 h and then with NGF-β (50 ng/ml) or EI-tPA (12 n
m
) for 10 min. B, PC12 cells were pretreated with MK-801 (1 μ
m
) for 2 h and then with EI-tPA (12 or 60 n
m
) for 10 min or 1 h. C, PC12 cells in which the NR1 subunit of the NMDA-R was silenced and cells transfected with NTC siRNA were treated with NGF-β (50 ng/ml), EI-tPA (12 n
m
), or vehicle for 10 min. Equal amounts of cellular protein (100 μg) were subjected to SDS-PAGE and immunoblot analysis to detect phosphorylated Trk (p-Trk). Blots were reprobed to detect β-actin as a loading control. Each blot is representative of at least three independent studies.
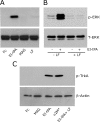
FIGURE 6.
LRP1-initiated cell signaling is ligand-specific. A, PC12 cells were treated with free Fc (20 n
m
), EI-tPA (12 n
m
), MAG (20 n
m
), or lactoferrin (20 n
m
) for 10 min. Cell extracts were subjected to immunoblot analysis to detect phosphorylated ERK1/2 (p-ERK) and total ERK1/2 (T-ERK). B, PC12 cells were pretreated with lactoferrin (250 n
m
) or vehicle for 1 h and then treated with vehicle or EI-tPA (12 n
m
) for 10 min. C, PC12 cells were treated with free Fc (20 n
m
), MAG (20 n
m
), EI-tPA (12 n
m
), α2M* (10 n
m
), or EI-tPA in the presence of 250 n
m
lactoferrin for 10 min. Cell extracts were subjected to immunoblot analysis to detect phosphorylated TrkA (p-TrkA) and β-actin as a loading control.
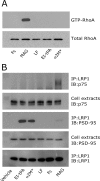
FIGURE 7.
p75NTR associates with LRP1 selectively in MAG-treated cells. A, N2a cells were incubated with free Fc (20 n
m
), MAG (20 n
m
), lactoferrin (20 n
m
), EI-tPA (12 n
m
), or α2M* (10 n
m
) for 10 min. GTP-bound RhoA was determined by affinity precipitation. The original cell extracts were studied by immunoblot analysis using the same antibody to determine total RhoA. B, N2a cells were incubated with vehicle (SFM), EI-tPA (12 n
m
), α2M* (10 n
m
), lactoferrin (20 n
m
), free Fc (20 n
m
), or MAG (20 n
m
) for 10 min. Extracts were prepared and adsorbed sequentially with nonspecific IgG coupled to Protein A-Sepharose and LRP1-specific antibody coupled to Protein A-Sepharose. Proteins that precipitated (IP) with LRP1-specific antibody were subjected to immunoblot analysis (IB) to detect p75NTR, PSD-95, and LRP1. The cell extracts from which the immunoprecipitates were prepared also were subjected to immunoblot analysis to detect PSD-95 and LRP1. The blots are representative of five independent experiments.
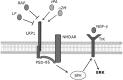
FIGURE 8.
Model showing LRP1, NMDA-R, and Trk receptor, functioning as a single system to activate cell signaling in response to LRP1 ligands. tPA and α2M* are shown as permissive ligands that activate cell signaling. RAP and lactoferrin (LF) bind to LRP1 but may antagonize cell signaling in response to permissive ligands. MAG binds with high affinity to LRP1 but apparently assembles an entirely different co-receptor network (not shown).
Similar articles
- PAI1 blocks NMDA receptor-mediated effects of tissue-type plasminogen activator on cell signaling and physiology.
Gonias SL, Banki MA, Gilder AS, Azmoon P, Campana WM, Mantuano E. Gonias SL, et al. J Cell Sci. 2018 Jul 26;131(14):jcs217083. doi: 10.1242/jcs.217083. J Cell Sci. 2018. PMID: 29930084 Free PMC article. - The LRP1/CD91 ligands, tissue-type plasminogen activator, α2-macroglobulin, and soluble cellular prion protein have distinct co-receptor requirements for activation of cell-signaling.
Mantuano E, Azmoon P, Banki MA, Gunner CB, Gonias SL. Mantuano E, et al. Sci Rep. 2022 Oct 20;12(1):17594. doi: 10.1038/s41598-022-22498-1. Sci Rep. 2022. PMID: 36266319 Free PMC article. - The functional role of the second NPXY motif of the LRP1 beta-chain in tissue-type plasminogen activator-mediated activation of N-methyl-D-aspartate receptors.
Martin AM, Kuhlmann C, Trossbach S, Jaeger S, Waldron E, Roebroek A, Luhmann HJ, Laatsch A, Weggen S, Lessmann V, Pietrzik CU. Martin AM, et al. J Biol Chem. 2008 May 2;283(18):12004-13. doi: 10.1074/jbc.M707607200. Epub 2008 Mar 5. J Biol Chem. 2008. PMID: 18321860 - Plasminogen activator receptor assemblies in cell signaling, innate immunity, and inflammation.
Gonias SL. Gonias SL. Am J Physiol Cell Physiol. 2021 Oct 1;321(4):C721-C734. doi: 10.1152/ajpcell.00269.2021. Epub 2021 Aug 18. Am J Physiol Cell Physiol. 2021. PMID: 34406905 Free PMC article. Review. - Tissue plasminogen activator in the amygdala: a new role for an old protease.
Skrzypiec AE, Buczko W, Pawlak R. Skrzypiec AE, et al. J Physiol Pharmacol. 2008 Dec;59 Suppl 8:135-46. J Physiol Pharmacol. 2008. PMID: 19258671 Review.
Cited by
- The activities of LDL Receptor-related Protein-1 (LRP1) compartmentalize into distinct plasma membrane microdomains.
Laudati E, Gilder AS, Lam MS, Misasi R, Sorice M, Gonias SL, Mantuano E. Laudati E, et al. Mol Cell Neurosci. 2016 Oct;76:42-51. doi: 10.1016/j.mcn.2016.08.006. Epub 2016 Aug 23. Mol Cell Neurosci. 2016. PMID: 27565578 Free PMC article. - A Soluble PrPC Derivative and Membrane-Anchored PrPC in Extracellular Vesicles Attenuate Innate Immunity by Engaging the NMDA-R/LRP1 Receptor Complex.
Mantuano E, Azmoon P, Banki MA, Sigurdson CJ, Campana WM, Gonias SL. Mantuano E, et al. J Immunol. 2022 Jan 1;208(1):85-96. doi: 10.4049/jimmunol.2100412. Epub 2021 Nov 22. J Immunol. 2022. PMID: 34810220 Free PMC article. - LRP1 modulates the microglial immune response via regulation of JNK and NF-κB signaling pathways.
Yang L, Liu CC, Zheng H, Kanekiyo T, Atagi Y, Jia L, Wang D, N'songo A, Can D, Xu H, Chen XF, Bu G. Yang L, et al. J Neuroinflammation. 2016 Dec 8;13(1):304. doi: 10.1186/s12974-016-0772-7. J Neuroinflammation. 2016. PMID: 27931217 Free PMC article. - Mesenchymal Stromal Cells Promote Axonal Outgrowth Alone and Synergistically with Astrocytes via tPA.
Qian JY, Chopp M, Liu Z. Qian JY, et al. PLoS One. 2016 Dec 13;11(12):e0168345. doi: 10.1371/journal.pone.0168345. eCollection 2016. PLoS One. 2016. PMID: 27959956 Free PMC article. - The NMDA receptor functions independently and as an LRP1 co-receptor to promote Schwann cell survival and migration.
Mantuano E, Lam MS, Shibayama M, Campana WM, Gonias SL. Mantuano E, et al. J Cell Sci. 2015 Sep 15;128(18):3478-88. doi: 10.1242/jcs.173765. Epub 2015 Aug 13. J Cell Sci. 2015. PMID: 26272917 Free PMC article.
References
- Collen D. (1987) Molecular mechanisms of fibrinolysis and their application to fibrin-specific thrombolytic therapy. J. Cell Biochem. 33, 77–86 - PubMed
- Nicole O., Docagne F., Ali C., Margaill I., Carmeliet P., MacKenzie E. T., Vivien D., Buisson A. (2001) The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat. Med. 7, 59–64 - PubMed
- Benchenane K., Berezowski V., Ali C., Fernández-Monreal M., López-Atalaya J. P., Brillault J., Chuquet J., Nouvelot A., MacKenzie E. T., Bu G., Cecchelli R., Touzani O., Vivien D. (2005) Tissue-type plasminogen activator crosses the intact blood-brain barrier by low density lipoprotein receptor-related protein-mediated transcytosis. Circulation 111, 2241–2249 - PubMed
- Baranes D., Lederfein D., Huang Y. Y., Chen M., Bailey C. H., Kandel E. R. (1998) Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron 21, 813–825 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous