Over-expression of an inactive mutant cathepsin D increases endogenous alpha-synuclein and cathepsin B activity in SH-SY5Y cells - PubMed (original) (raw)
. 2014 Mar;128(6):950-61.
doi: 10.1111/jnc.12497. Epub 2013 Nov 13.
Affiliations
- PMID: 24138030
- PMCID: PMC3951679
- DOI: 10.1111/jnc.12497
Over-expression of an inactive mutant cathepsin D increases endogenous alpha-synuclein and cathepsin B activity in SH-SY5Y cells
Donna Crabtree et al. J Neurochem. 2014 Mar.
Abstract
Parkinson's disease is a neurodegenerative movement disorder. The histopathology of Parkinson's disease comprises proteinaceous inclusions known as Lewy bodies, which contains aggregated α-synuclein. Cathepsin D (CD) is a lysosomal protease previously demonstrated to cleave α-synuclein and decrease its toxicity in both cell lines and mouse brains in vivo. Here, we show that pharmacological inhibition of CD, or introduction of catalytically inactive mutant CD, resulted in decreased CD activity and increased cathepsin B activity, suggesting a possible compensatory response to inhibition of CD activity. However, this increased cathepsin B activity was not sufficient to maintain α-synuclein degradation, as evidenced by the accumulation of endogenous α-synuclein. Interestingly, the levels of LC3, LAMP1, and LAMP2, proteins involved in autophagy-lysosomal activities, as well as total lysosomal mass as assessed by LysoTracker flow cytometry, were unchanged. Neither autophagic flux nor proteasomal activities differs between cells over-expressing wild-type versus mutant CD. These observations point to a critical regulatory role for that endogenous CD activity in dopaminergic cells in α-synuclein homeostasis which cannot be compensated for by increased Cathepsin B. These data support the potential need to enhance CD function in order to attenuate α-synuclein accumulation as a therapeutic strategy against development of synucleinopathy.
Keywords: autophagy; cathepsin D; lysosome; α-synuclein.
© 2013 International Society for Neurochemistry.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
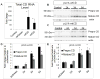
Figure 1. Increased CD mRNA and protein in SH-SY5Y cells transduced with lentivirus expressing either wild type or D295N mutant CD
A. RT-PCR of total CD transcripts in response to zsGreen, wild type CD, and D295N mutant CD lentivirus transduction 3d post-transduction versus control (non-transduced) cells. Shown is fold increase compared to control. Data = mean ± SEM (*p<0.05 control = zsGreen < wtCD = mtCD). B–C. Western blot showing CD levels after lentiviral infection with pLVX-zsGreen (control, B and C, 3 d after infection), pLVX-wtCD (B), or pLVX-mtCD (C) 2d, 3d, and 5d post-transduction. D. Quantification of western blot for pLVX-wtCD from panel B. Data = mean ± SEM (*p<0.001 zsGreen = 2d < 3d < 5d). E. Quantification of western blot for pLVX-mtCD from panel C. Data = mean ± SEM (*p<0.001 zsGreen = 2d < 3d = 5d). Each panel was a summary from experiments performed with cells from 3 independent transductions of each construct.
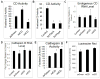
Figure 2. Levels of endogenous α-synuclein in cells over expressing wild type or D295N mutant CD
A. Western blot for α-synuclein levels in response to wild type and D295N mutant CD compared to zsGreen control. B. Corresponding quantification of band intensities. Data = mean ± SEM. (*p<0.05 zsGreen < mtCD). C. α-Synuclein mRNA level is unchanged by pLVX-mtCD D295N or pLVX-wtCD (_p_>0.05 for all comparisons). The experiments were performed with cells from 3 independent transductions of each construct. All extracts were collected 3d post-transduction.
Figure 3. Knockdown of cathepsin D did not affect alpha-synuclein levels
A. Western blot for prepro CD, mature CD, and α-synuclein levels following transfection with either 750 nM non-targeting or CD siRNA. B. Corresponding quantification of CD band intensities. Data = mean ± SEM. (*p<0.05 comparing CDsiRNA to non-target siRNA). C. Corresponding quantification of α-synuclein band intensities. Data = mean ± SEM. (*p<0.05 comparing CDsiRNA to non-target siRNA). All extracts were collected 3d post-transfection.
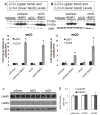
Figure 4. CD and CB activities in cells over expressing wild type or D295N mutant CD
A. CD activity is significantly elevated in response to wtCD and dramatically reduced in response to D295N mutant CD over expression. Data = mean ± SEM (*p<0.001 mtCD < zsGreen < wtCD). B. 100 μM PepA treatment in non-transduced cells causes a marked decrease in endogenous CD activity at 3d and 5d. Data = mean ± SEM. (*_p_<0.001 3d PepA = 5d PepA < control). C. RT-PCR of endogenous CD shows no statistically significant changes in response to any of the lentiviruses. Data = mean ± SEM (_p_>0.05 for all comparisons). D. Cathepsin B mRNA levels are unaffected by transduction with pLVX-zsGreen, pLVX-wtCD, or pLVX-mtCD. Data= mean ± SEM (_p_>0.05 for all comparisons). E. Cathepsin B activity is increased in response to wtCD but even more so in response to mtCD. Data = mean ± SEM (*p<0.001 zsGreen < wtCD < mtCD). F. Flow cytometry analyses of LysoTracker Red staining in zsGreen, wtCD and mtCD transduced cells. Quantification shows no significant difference among these extracts. Each panel was a summary from experiments performed with cells from 3 independent transductions of each construct.
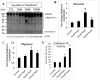
Figure 5. Autophagic flux analyses in cells over expressing wild type or D295N mutant CD
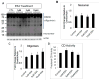
A–B. LC3 western blot after treatment of zsGreen (control, A and B), wtCD (A), and mtCD (B) with and without 10 nM BafA1 for 24 hr. Quantification of LC3-I and LC3-II in response to wtCD (A, lower panel) and mtCD (B, lower panel) shows LC3-II is significantly increased in response to addition of BafA1. Data = mean ± SEM. (*p<0.001 zsGreen = wtCD < zsGreen+Baf = wtCD+Baf and *p<0.001 zsGreen = mtCD < zsGreen+BafA1 = mtCD+BafA1). C. Western blot analyses of LAMP-1 and LAMP-2 in lysates from zsGreen, wtCD and mtCD transduced cells. D. Quantification of (C) shows no significant difference among these extracts. Each panel was a summary from experiments performed with cells from 3 independent transductions of each construct.
Figure 6. Pepstatin A-induced inhibition of CD activity leads to similar cellular changes as does transduction with pLVX-mtCD
A. Western blot showing α-synuclein levels in response to control, 10μM, 50μM, and 100μM PepA. B. Quantification of monomeric α-synuclein (shown in panel A). Data = mean ± SEM. (*p<0.05 control = 10 μM < 50 μM = 100 μM). C. Quantification of α-synuclein HMW species (shown in panel A). Data = mean ± SEM. (**p<0.001 control = 10 μM < 50 μM; *p<0.05 control = 10 μM < 100 μM; #p<0.05 100 μM < 50 μM). D. Cathepsin B activity is increased in a dose-dependent fashion in response to PepA-induced CD inhibition. Data = mean ± SEM. (*p<0.01 control = 10 μM < 50 μM < 100 μM).
Figure 7. Cathepsin B inhibition by E64 does not lead to α-synuclein accumulation
A. Western blot for α-synuclein levels following 1 μM, 5 μM, or 10 μM E64 treatment for 3d compared to control group. Quantification shows that α-synuclein levels are not significantly changed by any dose of E64 in the monomeric (B), or HMW (C) forms. (_p_>0.05 for all comparisons). D. CD activity level is not altered in response to E64-induced cathepsin B inhibition. (_p_>0.05 for all comparisons).
Figure 8. E64 but not PepA Treatment Results in a Dose-Dependent Increase in LC3-II Levels
A. Western blot of LC3 levels in response to PepA. Lower panel shows quantification. Data = mean ± SEM (*p<0.001 for LC3-I 100μM < all others). B. Western blot of LC3 levels in response to E64. Lower panel shows quantifications. Data = mean ± SEM (*p<0.001 for LC3-II control = 1 μM < 5 μM < 10 μM). Chloroquine (CQ) treatment was used as a positive control. Each panel was a summary from experiments performed with cells from 3 independent drug treatments.
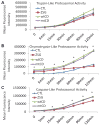
Figure 9. Proteasomal activities in cells over expressing wild type or D295N mutant CD
Trypsin-like (A), chymotrypsin-like (B), and caspase-like (C) proteasomal activities in lysates from non-transduced, pLVX-zsGreen, pLVX-wtCD and p-LVX-mtCD transduced cells. Fluorescent products were monitored at 0, 5, 15, 30, 60, 90, and 120 min. A. Trypsin-like proteasome activity was not significantly different as a result of increased CD expression and activity. Data = mean ± SEM (p>0.05 for all comparisons). B. Chymotrypsin-like activity was significantly greater in wtCD and mtCD samples than in zsGreen and non-transduced controls. Data = mean ± SEM (*p<0.001 CTL=zsGreen < wtCD = mtCD). C. Caspase-like activity was also greater in both wtCD and mtCD over expressing cells beginning at 15 min and continuing through the last measured time point (120 min). Data = mean ± SEM (*p<0.001 CTL = zsGreen < wtCD = mtCD). Each panel was a summary from experiments performed with cells from 3 independent transductions of each virus.
Similar articles
- Recombinant pro-CTSD (cathepsin D) enhances SNCA/α-Synuclein degradation in α-Synucleinopathy models.
Prieto Huarcaya S, Drobny A, Marques ARA, Di Spiezio A, Dobert JP, Balta D, Werner C, Rizo T, Gallwitz L, Bub S, Stojkovska I, Belur NR, Fogh J, Mazzulli JR, Xiang W, Fulzele A, Dejung M, Sauer M, Winner B, Rose-John S, Arnold P, Saftig P, Zunke F. Prieto Huarcaya S, et al. Autophagy. 2022 May;18(5):1127-1151. doi: 10.1080/15548627.2022.2045534. Epub 2022 Apr 28. Autophagy. 2022. PMID: 35287553 Free PMC article. - Cysteine cathepsins are essential in lysosomal degradation of α-synuclein.
McGlinchey RP, Lee JC. McGlinchey RP, et al. Proc Natl Acad Sci U S A. 2015 Jul 28;112(30):9322-7. doi: 10.1073/pnas.1500937112. Epub 2015 Jul 13. Proc Natl Acad Sci U S A. 2015. PMID: 26170293 Free PMC article. - Downregulation of Protease Cathepsin D and Upregulation of Pathologic α-Synuclein Mediate Paucity of DNAJC6-Induced Degeneration of Dopaminergic Neurons.
Chiu CC, Chen YL, Weng YH, Liu SY, Li HL, Yeh TH, Wang HL. Chiu CC, et al. Int J Mol Sci. 2024 Jun 18;25(12):6711. doi: 10.3390/ijms25126711. Int J Mol Sci. 2024. PMID: 38928416 Free PMC article. - The vicious cycle between α-synuclein aggregation and autophagic-lysosomal dysfunction.
Bellomo G, Paciotti S, Gatticchi L, Parnetti L. Bellomo G, et al. Mov Disord. 2020 Jan;35(1):34-44. doi: 10.1002/mds.27895. Epub 2019 Nov 15. Mov Disord. 2020. PMID: 31729779 Review. - Ubiquitination of alpha-synuclein and autophagy in Parkinson's disease.
Engelender S. Engelender S. Autophagy. 2008 Apr;4(3):372-4. doi: 10.4161/auto.5604. Epub 2008 Jan 18. Autophagy. 2008. PMID: 18216494 Review.
Cited by
- A(a)LS: Ammonia-induced amyotrophic lateral sclerosis.
Parekh B. Parekh B. F1000Res. 2015 May 14;4:119. doi: 10.12688/f1000research.6364.1. eCollection 2015. F1000Res. 2015. PMID: 27785351 Free PMC article. - Autophagy in the Neuronal Ceroid Lipofuscinoses (Batten Disease).
Kim WD, Wilson-Smillie MLDM, Thanabalasingam A, Lefrancois S, Cotman SL, Huber RJ. Kim WD, et al. Front Cell Dev Biol. 2022 Feb 16;10:812728. doi: 10.3389/fcell.2022.812728. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35252181 Free PMC article. Review. - New multienzymatic complex formed between human cathepsin D and snake venom phospholipase A2.
Moraes JDN, Francisco AF, Dill LM, Diniz RS, de Oliveira CS, da Silva TMR, Caldeira CADS, Corrêa EA, Coutinho-Neto A, Zanchi FB, Fontes MRM, Soares AM, Calderon LA. Moraes JDN, et al. J Venom Anim Toxins Incl Trop Dis. 2022 Nov 4;28:e20220002. doi: 10.1590/1678-9199-JVATITD-2022-0002. eCollection 2022. J Venom Anim Toxins Incl Trop Dis. 2022. PMID: 36404954 Free PMC article. - Dysfunction of Cellular Proteostasis in Parkinson's Disease.
Lehtonen Š, Sonninen TM, Wojciechowski S, Goldsteins G, Koistinaho J. Lehtonen Š, et al. Front Neurosci. 2019 May 10;13:457. doi: 10.3389/fnins.2019.00457. eCollection 2019. Front Neurosci. 2019. PMID: 31133790 Free PMC article. Review. - Fibrillar α-synuclein toxicity depends on functional lysosomes.
Guiney SJ, Adlard PA, Lei P, Mawal CH, Bush AI, Finkelstein DI, Ayton S. Guiney SJ, et al. J Biol Chem. 2020 Dec 18;295(51):17497-17513. doi: 10.1074/jbc.RA120.013428. J Biol Chem. 2020. PMID: 33453994 Free PMC article.
References
- Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, Schapira AH. Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol. 2010;67:1464–1472. - PubMed
- Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch EC, Agid Y. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol Histopathol. 1997;12:25–31. - PubMed
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 NS047466/NS/NINDS NIH HHS/United States
- P30 AR48311/AR/NIAMS NIH HHS/United States
- R01 NS064090/NS/NINDS NIH HHS/United States
- P30 AR048311/AR/NIAMS NIH HHS/United States
- R01-NS064090/NS/NINDS NIH HHS/United States
- P30-NS047466/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous