Transdermal application of methimazole in hyperthyroid cats: a long-term follow-up study - PubMed (original) (raw)
Transdermal application of methimazole in hyperthyroid cats: a long-term follow-up study
Felicitas S Boretti et al. J Feline Med Surg. 2014 Jun.
Abstract
Transdermal methimazole is suggested as an alternative to oral therapy for hyperthyroid cats that are difficult to pill. However, no information on long-term management with this treatment is available. Our objective was therefore to retrospectively evaluate the efficacy and safety of long-term transdermal methimazole treatment in hyperthyroid cats. Sixty cats with newly diagnosed hyperthyroidism and available long-term follow-up information were included. Methimazole was formulated in a pluronic lecithin organogel-based vehicle and was applied to the pinna of the inner ear. Cats were re-evaluated at regular intervals. Median (range) follow-up was 22.6 months (3.6-88.4 months). Clinical improvement was observed in all cats and side effects were rare (mild transient gastrointestinal signs: n = 3; erythema of the pinna: n = 2, necessitating a switch to oral medication). Despite a significant decrease, with median T4 concentrations within the reference interval during the follow-up period, several cats repeatedly had T4 concentrations in the thyrotoxic and hypothyroid range. Maximal and minimal daily doses during the follow-up period were 15.0 and 1.0 mg, respectively; they were significantly higher than the starting dose after 24-36 months of therapy. Although the majority of owners were highly satisfied with the treatment, several admitted not treating their cat regularly. Transdermal methimazole is a safe option for the long-term management of feline hyperthyroidism. However, it seems difficult to keep the T4 concentrations constantly within the reference interval. Higher doses can be expected after prolonged treatment and, despite the convenience of transdermal application, owner compliance should be assessed regularly.
© ISFM and AAFP 2013.
Conflict of interest statement
The authors do not have any potential conflicts of interest to declare.
Figures
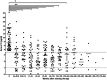
Figure 1
Scatter plot of serum T4 concentrations before and at different time points during long-term transdermal methimazole therapy. The median is indicated by a horizontal line. The horizontal dotted lines represent the reference T4 concentrations. *_P_Wilcoxon = 0.0004; **_P_Wilcoxon <0.0001
Similar articles
- Duration of t4 suppression in hyperthyroid cats treated once and twice daily with transdermal methimazole.
Boretti FS, Sieber-Ruckstuhl NS, Schäfer S, Baumgartner C, Riond B, Hofmann-Lehmann R, Reusch CE. Boretti FS, et al. J Vet Intern Med. 2013 Mar-Apr;27(2):377-81. doi: 10.1111/jvim.12040. Epub 2013 Feb 9. J Vet Intern Med. 2013. PMID: 23398124 - Transdermal methimazole treatment in cats with hyperthyroidism.
Hoffmann G, Marks SL, Taboada J, Hosgood GL, Wolfsheimer KJ. Hoffmann G, et al. J Feline Med Surg. 2003 Apr;5(2):77-82. doi: 10.1016/S1098-612X(02)00095-5. J Feline Med Surg. 2003. PMID: 12670432 Free PMC article. - Efficacy and safety of transdermal methimazole in the treatment of cats with hyperthyroidism.
Sartor LL, Trepanier LA, Kroll MM, Rodan I, Challoner L. Sartor LL, et al. J Vet Intern Med. 2004 Sep-Oct;18(5):651-5. doi: 10.1892/0891-6640(2004)18<651:easotm>2.0.co;2. J Vet Intern Med. 2004. PMID: 15515580 - Pharmacologic management of feline hyperthyroidism.
Trepanier LA. Trepanier LA. Vet Clin North Am Small Anim Pract. 2007 Jul;37(4):775-88, vii. doi: 10.1016/j.cvsm.2007.03.004. Vet Clin North Am Small Anim Pract. 2007. PMID: 17619011 Review. - Best practice for the pharmacological management of hyperthyroid cats with antithyroid drugs.
Daminet S, Kooistra HS, Fracassi F, Graham PA, Hibbert A, Lloret A, Mooney CT, Neiger R, Rosenberg D, Syme HM, Villard I, Williams G. Daminet S, et al. J Small Anim Pract. 2014 Jan;55(1):4-13. doi: 10.1111/jsap.12157. Epub 2013 Dec 27. J Small Anim Pract. 2014. PMID: 24372075 Review.
Cited by
- Efficacy of intramuscular hydroxocobalamin supplementation in cats with cobalamin deficiency and gastrointestinal disease.
Kook PH, Melliger RH, Hersberger M. Kook PH, et al. J Vet Intern Med. 2020 Sep;34(5):1872-1878. doi: 10.1111/jvim.15865. Epub 2020 Aug 20. J Vet Intern Med. 2020. PMID: 32815652 Free PMC article. - Pharmacokinetics of furosemide after intravenous, oral and transdermal administration to cats.
Sleeper MM, O'Donnell P, Fitzgerald C, Papich MG. Sleeper MM, et al. J Feline Med Surg. 2019 Oct;21(10):882-886. doi: 10.1177/1098612X18805879. Epub 2018 Oct 19. J Feline Med Surg. 2019. PMID: 30339054 Free PMC article. - Management and monitoring of hyperthyroid cats: a survey of Australian veterinarians.
Kopecny L, Higgs P, Hibbert A, Malik R, Harvey AM. Kopecny L, et al. J Feline Med Surg. 2017 Jun;19(6):559-567. doi: 10.1177/1098612X16634392. Epub 2016 Mar 10. J Feline Med Surg. 2017. PMID: 26965675 Free PMC article. - Prospective crossover clinical trial comparing transdermal with oral phenobarbital administration in epileptic cats.
Barnes Heller HL, Trepanier LA, Robertson M, Mei C. Barnes Heller HL, et al. J Feline Med Surg. 2019 Dec;21(12):1181-1187. doi: 10.1177/1098612X18823577. Epub 2019 Jan 28. J Feline Med Surg. 2019. PMID: 30688552 Free PMC article. - Evaluation of Antioxidant Supplementation on Redox Unbalance in Hyperthyroid Cats Treated with Methimazole: A Blinded Randomized Controlled Trial.
Candellone A, Badino P, Gianella P, Girolami F, Raviri G, Saettone V, Meineri G. Candellone A, et al. Antioxidants (Basel). 2019 Dec 23;9(1):15. doi: 10.3390/antiox9010015. Antioxidants (Basel). 2019. PMID: 31877998 Free PMC article.
References
- Peterson ME, Aucoin DP. Comparison of the disposition of carbimazole and methimazole in clinically normal cats. Res Vet Sci 1993; 54: 351–355. - PubMed
- Mooney CT. Feline hyperthyroidism. Diagnostics and therapeutics. Vet Clin North Am Small Anim Pract 2001; 31: 963–983. - PubMed
- Feldmann EC, Nelson RW. Feline hyperthyroidism (thyrotoxicosis). In: Feldmann EC, Nelson RW. (eds). Canine and feline endocrinology and reproduction. 3rd ed. St Louis, MO: Saunders, 2004, p 162.
- Peterson ME. Radioiodine treatment of hyperthyroidism. Clin Tech Small Anim Pract 2006; 21: 34–39. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous