Genetically engineered immunomodulatory Streptococcus thermophilus strains producing antioxidant enzymes exhibit enhanced anti-inflammatory activities - PubMed (original) (raw)
Genetically engineered immunomodulatory Streptococcus thermophilus strains producing antioxidant enzymes exhibit enhanced anti-inflammatory activities
Silvina Del Carmen et al. Appl Environ Microbiol. 2014 Feb.
Abstract
The aims of this study were to develop strains of lactic acid bacteria (LAB) having both immunomodulatory and antioxidant properties and to evaluate their anti-inflammatory effects both in vitro, in different cellular models, and in vivo, in a mouse model of colitis. Different Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus strains were cocultured with primary cultures of mononuclear cells. Analysis of the pro- and anti-inflammatory cytokines secreted by these cells after coincubation with candidate bacteria revealed that L. delbrueckii subsp. bulgaricus CRL 864 and S. thermophilus CRL 807 display the highest anti-inflammatory profiles in vitro. Moreover, these results were confirmed in vivo by the determination of the cytokine profiles in large intestine samples of mice fed with these strains. S. thermophilus CRL 807 was then transformed with two different plasmids harboring the genes encoding catalase (CAT) or superoxide dismutase (SOD) antioxidant enzymes, and the anti-inflammatory effects of recombinant streptococci were evaluated in a mouse model of colitis induced by trinitrobenzenesulfonic acid (TNBS). Our results showed a decrease in weight loss, lower liver microbial translocation, lower macroscopic and microscopic damage scores, and modulation of the cytokine production in the large intestines of mice treated with either CAT- or SOD-producing streptococci compared to those in mice treated with the wild-type strain or control mice without any treatment. Furthermore, the greatest anti-inflammatory activity was observed in mice receiving a mixture of both CAT- and SOD-producing streptococci. The addition of L. delbrueckii subsp. bulgaricus CRL 864 to this mixture did not improve their beneficial effects. These findings show that genetically engineering a candidate bacterium (e.g., S. thermophilus CRL 807) with intrinsic immunomodulatory properties by introducing a gene expressing an antioxidant enzyme enhances its anti-inflammatory activities.
Figures
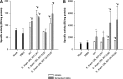
FIG 1
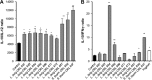
Cytokine profile after coincubation of LAB with primary cultures of mononuclear cells. (A) IL-10 and IL-12 cytokines were quantified by ELISA after coincubation of the different LAB with PBMC for 24 h, and IL-10/IL-12 ratios were determined; PBS was used a negative control. (B) IL-10 and IFN-γ cytokines were quantified by ELISA after coincubation of each individual strain grown in milk, yogurt (positive control), or unfermented milk (negative control) with Peyer's patch mononuclear cells for 24 h, and IL-10/IFN-γ ratios were determined. Results are expressed as means ± standard deviation (SD). Means with asterisks differ significantly from control values (*, P < 0.05; **, P < 0.01).
FIG 2
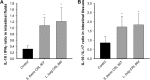
Evaluation of anti-inflammatory potential effects of selected lactobacilli and streptococci in vivo. The ratio of anti-inflammatory cytokines to proinflammatory cytokines induced in large intestine fluids (A) or cells in the intestinal tissues (B) of mice fed with unfermented milk (negative control) or with milk fermented by S. thermophilus CRL 807 or L. delbrueckii subsp. bulgaricus CRL 864 were determined. Results are expressed as means of ± SD (n = 9). Means with asterisks differ significantly from control values (*, P < 0.01).
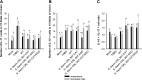
FIG 3
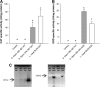
Detection of enzymatic activity and confirmation of recombinant streptococci. (A and B) Catalase (A) or superoxide dismutase (B) specific to WT S. thermophilus CRL 807, CRL807:CAT (S. thermophilus CRL 807 transformed with the pIL253-mnkat plasmid) or S. thermophilus CRL 807:SOD (S. thermophilus CRL 807 transformed with the pIL253-sodA plasmid) grown in LAPTg medium is expressed as enzymatic units (U) per mg of proteins and compared to the enzymatic activity of L. casei BL23 harboring the same plasmid (L. casei BL23:CAT and BL23:SOD). The control is the culture medium. Results are expressed as means ± SD. Means without a common letter differ significantly (P < 0.05). (C) PCR confirming single bands of approximately 600 and 1,200 bp that correspond to the CAT and SOD enzymes, respectively, in the recombinant S. thermophilus CRL 807 (807:CAT and 807:SOD) and in the L. casei BL23 positive controls harboring the same plasmid (BL23:CAT and BL23:SOD). No amplification was observed in the WT strains.
FIG 4
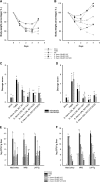
Effects of recombinant streptococci expressing antioxidant enzymes on TNBS-induced inflammation in mice. The body weight percentage (A and B), macro- and microscopic damage scores (C and D), and liver microbial translocation (E and F) were evaluated in mice from the mock group, mice from the TNBS group, and mice that received the WT (S. thermophilus CRL 807 WT), S. thermophilus CRL 807:CAT (S. thermophilus CRL807 transformed with the pIL253-mnkat plasmid), S. thermophilus CRL 807:SOD (S. thermophilus CRL 807 transformed with the pIL253-sodA plasmid), or S. thermophilus CRL 807:CAT/SOD (coadministration of both recombinant streptococci) in suspension (A, C, and D) or in fermented milk (B, D, and F). Body weight was measured from the day of TNBS inoculation up to 4 days after TNBS inoculation and they is represented as a percentage of the initial mouse body weight. Microscopic (black bars) and macroscopic (gray bars) damage scores correspond to samples taken 4 days after TNBS inoculation. Each value represents the mean ± SD (n = 9). Microbial growth in MacConkey, MRS, or LAPTg of liver samples obtained from different groups was evaluated. Results are expressed as means ± SD of the log CFU/g liver. Means for each value without a common letter differ significantly (P < 0.05).
FIG 5
Enzymatic activity in the large intestine contents. CAT (A) and SOD (B) specific activities were determined in the intestinal contents of mice from the mock group, mice from the TNBS group, and mice that received the WT (S. thermophilus CRL 807 WT), S. thermophilus CRL 807:CAT (S. thermophilus CRL 807transformed with the pIL253-mnkat plasmid), S. thermophilus CRL 807:SOD (S. thermophilus CRL 807 transformed with the pIL253-sodA plasmid), or S. thermophilus CRL 807:CAT/SOD (coadministration of both recombinant streptococci) in suspension (white bars) or in fermented milk (gray bars). The results are expressed as the means of the enzymatic units (EU) per mg of protein. * and #, significantly different from the mock or TNBS group, respectively (P < 0.01).
FIG 6
IL-17- and IL-10-producing cells in the large intestine tissues. (A and B) IL-17 (A)- and IL-10 (B)-positive cells were evaluated by immunofluorescence in mice from the mock group, mice from the TNBS group, and mice that received the WT (S. thermophilus CRL 807 WT), S. thermophilus CRL807:CAT (S. thermophilus CRL807 transformed with the pIL253-mnkat plasmid), S. thermophilus CRL 807:SOD (S. thermophilus CRL 807 transformed with the pIL253-sodA plasmid), or S. thermophilus CRL 807:CAT/SOD (coadministration of both recombinant streptococci) in suspension (black bars) or in fermented milk (gray bars). The results are expressed as the means of the total number of positive cells counted in 10 fields at a magnification of ×1,000. (C) Ratio between the number of IL-10+ cells and the number of IL-17+ cells for each group. Data correspond to the means ± SD (n = 9). Means for each value without a common letter differ significantly (P < 0.01).
Similar articles
- Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn's disease in mice.
LeBlanc JG, del Carmen S, Miyoshi A, Azevedo V, Sesma F, Langella P, Bermúdez-Humarán LG, Watterlot L, Perdigon G, de Moreno de LeBlanc A. LeBlanc JG, et al. J Biotechnol. 2011 Feb 10;151(3):287-93. doi: 10.1016/j.jbiotec.2010.11.008. Epub 2010 Dec 16. J Biotechnol. 2011. PMID: 21167883 - Enhancing the Sweetness of Yoghurt through Metabolic Remodeling of Carbohydrate Metabolism in Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus.
Sørensen KI, Curic-Bawden M, Junge MP, Janzen T, Johansen E. Sørensen KI, et al. Appl Environ Microbiol. 2016 May 31;82(12):3683-3692. doi: 10.1128/AEM.00462-16. Print 2016 Jun 15. Appl Environ Microbiol. 2016. PMID: 27107115 Free PMC article. - Effect of riboflavin-producing bacteria against chemically induced colitis in mice.
Levit R, Savoy de Giori G, de Moreno de LeBlanc A, LeBlanc JG. Levit R, et al. J Appl Microbiol. 2018 Jan;124(1):232-240. doi: 10.1111/jam.13622. Epub 2017 Dec 18. J Appl Microbiol. 2018. PMID: 29080295 - Advances in Genetic Tools and Their Application in Streptococcus thermophilus.
Zhao R, Chen Z, Liang J, Dou J, Guo F, Xu Z, Wang T. Zhao R, et al. Foods. 2023 Aug 19;12(16):3119. doi: 10.3390/foods12163119. Foods. 2023. PMID: 37628118 Free PMC article. Review.
Cited by
- Neuroprotective Effects Exerted by a Combination of Selected Lactic Acid Bacteria in a Mouse Parkinsonism Model under Levodopa-Benserazide Treatment.
Pérez Visñuk D, LeBlanc JG, de Moreno de LeBlanc A. Pérez Visñuk D, et al. Neurochem Res. 2024 Oct;49(10):2940-2956. doi: 10.1007/s11064-024-04217-6. Epub 2024 Aug 1. Neurochem Res. 2024. PMID: 39088165 - Preventing Colitis-Associated Colon Cancer With Antioxidants: A Systematic Review.
Irrazabal T, Thakur BK, Croitoru K, Martin A. Irrazabal T, et al. Cell Mol Gastroenterol Hepatol. 2021;11(4):1177-1197. doi: 10.1016/j.jcmgh.2020.12.013. Epub 2021 Jan 5. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33418102 Free PMC article. - Delivery of IL-35 by Lactococcus lactis Ameliorates Collagen-Induced Arthritis in Mice.
Maddaloni M, Kochetkova I, Hoffman C, Pascual DW. Maddaloni M, et al. Front Immunol. 2018 Nov 20;9:2691. doi: 10.3389/fimmu.2018.02691. eCollection 2018. Front Immunol. 2018. PMID: 30515168 Free PMC article. - Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets.
Santana PT, Rosas SLB, Ribeiro BE, Marinho Y, de Souza HSP. Santana PT, et al. Int J Mol Sci. 2022 Mar 23;23(7):3464. doi: 10.3390/ijms23073464. Int J Mol Sci. 2022. PMID: 35408838 Free PMC article. Review. - Probiotics and Postbiotics as an Alternative to Antibiotics: An Emphasis on Pigs.
Ali MS, Lee EB, Hsu WH, Suk K, Sayem SAJ, Ullah HMA, Lee SJ, Park SC. Ali MS, et al. Pathogens. 2023 Jun 26;12(7):874. doi: 10.3390/pathogens12070874. Pathogens. 2023. PMID: 37513721 Free PMC article. Review.
References
- LeBlanc JG, de Moreno de LeBlanc A. 2013. Crohn's disease: etiology, diagnosis and treatment options, 1st ed. Nova Science Publishers, Inc., Hauppauge, NY
- FAO/WHO 2001. Expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Cordoba, Argentina, 1 to 4 October 2001 WHO, Geneva, Switzerland
- LeBlanc JG, del Carmen S, Zurita Turk M, Alvarenga Lima F, Santos Pontes D, Miyoshi A, Azevedo V, de Moreno de LeBlanc A. 2013. Mechanisms involved in the anti-inflammatory properties of native and genetically engineered lactic acid bacteria. Anti-Infect. Agents 11:59–69. 10.2174/10059 - DOI
- Sheil B, Shanahan F, O'Mahony L. 2007. Probiotic effects on inflammatory bowel disease. J. Nutr. 137:819S–824S - PubMed
- de Moreno de LeBlanc A, Chaves S, Perdigon G. 2009. Effect of yoghurt on the cytokine profile using a murine model of intestinal inflammation. Eur. J. Inflam. 7:97–109
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous