Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/β-catenin signal pathway - PubMed (original) (raw)
Clinical Trial
Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/β-catenin signal pathway
Qing Ji et al. PLoS One. 2013.
Abstract
Resveratrol, extracted from Chinese herbal medicine Polygonum cuspidatum, is known to inhibit invasion and metastasis of human colorectal cancer (CRC), in which long non-coding Metastasis Associated Lung Adenocarcinoma Transcript 1 (RNA-MALAT1) also plays an important role. Using MALAT1 lentiviral shRNA and over-expression constructs in CRC derived cell lines, LoVo and HCT116, we demonstrated that the anti-tumor effects of resveratrol on CRC are through inhibiting Wnt/β-catenin signaling, thus the expression of its target genes such as c-Myc, MMP-7, as well as the expression of MALAT1. In detail, resveratrol down-regulates MALAT1, resulting in decreased nuclear localization of β-catenin thus attenuated Wnt/β-catenin signaling, which leads to the inhibition of CRC invasion and metastasis. This finding of ours surely provides important pre-clinical evidence supporting future use of resveratrol in prevention and treatment of CRC.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
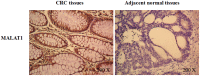
Figure 1. Overexpression of MALAT1 in human colorectal cancer tissues.
In situ hybridization was applied to investigate the MALAT1 expression in 60 paraffin-embedded tumor tissue and adjacent normal tissue samples of CRC patients.
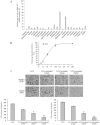
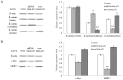
Figure 2. Resveratrol inhibites the proliferation, migration and invasion of CRC LoVo cells.
(A): Twenty-two candidate Chinese medicine monomer anticancer drugs were tested for the effective inhibition doses on the MALAT1 expression in LoVo cells. (B): Correlation of resveratrol drug doses and growth inhibition in LoVo cells. Y axis: percentage of growth inhibition; X axis: resveratrol concentrations (µM). (C): Assessment and quantification of cell migration and invasion of LoVo cells. Values represent the number of migratory/invasive cells per 5 high power fields. *p<0.05 or **p<0.01, compared with control LoVo cells.
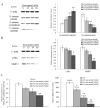
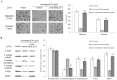
Figure 3. Resveratrol decreases the nuclear localization of β-catenin, levels of its downstream proteins, and represses MALAT1 expression in LoVo cells.
(A): Total or cell extracts of different cellular compartments from LoVo cells treated with resveratrol were probed for β-catenin. β-catenin protein levels were quantified. *p<0.05 or **p<0.01, compared with control group. (B): Total cell extracts of LoVo cells treated with resveratrol were probed for c-Myc, MMP-7 and the protein levels were quantified. *p<0.05 or **p<0.01, compared with control LoVo cells. (C): The expression levels of MALAT1 from LoVo cells treated with indicated doses of resveratrol were determined by real time PCR. The relative MALAT1 promoter activities in LoVo cells treated with indicated resveratrol doses were measured. *p<0.05 or **p<0.01, compared with control LoVo cells.
Figure 4. MALAT1 promotes the proliferation, invasion and migration of LoVo cells.
(A): In vitro growth of LoVo cells expressing MALAT1-shRNA, MALAT1-overexpression, empty vector vs. parental cells. (B): Assessment and quantification of cell migration and invasion of indicated LoVo cells in A. Values represent the number of migratory/invasive cells per 5 high power fields. *p<0.05 or **p<0.01, compared with control LoVo group.
Figure 5. MALAT1 increases the nuclear localization of β-catenin and levels of its downstream proteins in LoVo cells.
(A): Whole or cell extracts of different cellular compartments from LoVo cells expressing MALAT1-shRNA, MALAT1-overexpression, empty vector were probed for β-catenin and the protein levels were quantified. *p<0.05 or **p<0.01, compared with vector control. (B): Whole or cell extracts of different cellular compartments from LoVo cells expressing MALAT1-shRNA, MALAT1-overexpressed, empty vector were probed for c-Myc, MMP-7 and the protein levels were quantified. *p<0.05 or **p<0.01, compared with vector control.
Figure 6. Overexpression of MALAT1 attenuates the suppressive effect of resveratrol on migration and invasion in LoVo cells.
(A): LoVo cells expressing MALAT1-overexpressed, empty vector were treated with or without 50 µM resveratrol for 48 hours and cellular migration and invasion ability were measured. (B): Whole or cell extracts of different cellular compartments from LoVo cells expressing MALAT1-overexpressed, empty vector treated with or without 50 µM resveratrol were probed for β-catenin, c-Myc, MMP-7, and the protein levels were quantified. *p<0.05 or **p<0.01, compared with LoVo cells treated with 50 µM resveratrol.
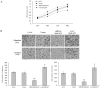
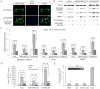
Figure 7. Resveratrol inhibits the Wnt/β-catenin signaling through regulating MALAT1.
(A): Resveratrol negated LiCl induced nuclear translocation of β-catenin. Immunofluorescence staining of β-catenin in LoVo cells treated with LiCl, with or without resveratrol. (B): Nuclear extracts from LoVo cells expressing MALAT1-shRNA, MALAT1-overexpressed, empty vector, treated with LiCl with or without resveratrol, were probed for β-catenin, c-Myc, MMP-7. (C): Quantification of β-catenin, c-Myc, MMP-7 protein levels from the data shown in (A). **p<0.01, compared with blank group or LiCl alone group. (D): The relative LEF/TCF promoter activities in LoVo cells expressing MALAT1-shRNA, MALAT1-overexpressed, empty vector, treated by LiCl, with or without resveratrol. **p<0.01, compared with blank group or LiCl alone group. (E): No interaction between MALAT1 and β-catenin. Insert: RT-PCR result showing amount of RNA pulled-down by RNA-binding protein immunoprecipitation. As negative controls, no cDNA (blank) or IgG alone was used. Lower pane: quantification result of the insert.
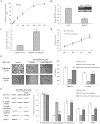
Figure 8. Resveratrol inhibits the Wnt/β-catenin signaling through regulating MALAT1.in CRC HCT116 cells.
(A): Resveratrol inhibited the proliferation of HCT116 cells with an IC50 of 100 µM. (B): HCT 116 cells showed lower MALAT1 expression than LoVo cells. **p<0.01, compared with LoVo cells. (C): Overexpression of MALAT1 in HCT116 cells. (D): Overexpression of MALAT1 promoted the in vitro growth of HCT116 cells. (E): Overexpression of MALAT1 attenuated the suppressive effect of resveratrol on migration and invasion in HCT116 cells. HCT116 cells expressing MALAT1-overexpressed, empty vector were treated with or without 50 µM resveratrol for 48 hours and cellular migration and invasion ability were measured. (F): Whole or cell extracts of different cellular compartments from HCT116 cells expressing MALAT1-overexpressed, empty vector treated with or without 50 µM resveratrol for 48 hours were probed for β-catenin, c-Myc, MMP-7 and the protein levels were quantified. *p<0.05 or **p<0.01, compared with HCT116 cells treated with 100 µM resveratrol.
Similar articles
- Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression.
Ji Q, Liu X, Han Z, Zhou L, Sui H, Yan L, Jiang H, Ren J, Cai J, Li Q. Ji Q, et al. BMC Cancer. 2015 Mar 5;15:97. doi: 10.1186/s12885-015-1119-y. BMC Cancer. 2015. PMID: 25884904 Free PMC article. - The long noncoding RNA BC0209135 inhibits the cell invasion through Wnt/β-catenin signaling in colorectal cancer.
Zheng Q, Lin Y, Chen P, Fan YP. Zheng Q, et al. Eur Rev Med Pharmacol Sci. 2018 Jun;22(12):3763-3770. doi: 10.26355/eurrev_201806_15258. Eur Rev Med Pharmacol Sci. 2018. PMID: 29949151 - JMJD2C promotes colorectal cancer metastasis via regulating histone methylation of MALAT1 promoter and enhancing β-catenin signaling pathway.
Wu X, Li R, Song Q, Zhang C, Jia R, Han Z, Zhou L, Sui H, Liu X, Zhu H, Yang L, Wang Y, Ji Q, Li Q. Wu X, et al. J Exp Clin Cancer Res. 2019 Oct 29;38(1):435. doi: 10.1186/s13046-019-1439-x. J Exp Clin Cancer Res. 2019. PMID: 31665047 Free PMC article. - MALAT1: A Promising Therapeutic Target for the Treatment of Metastatic Colorectal Cancer.
Uthman YA, Ibrahim KG, Abubakar B, Bello MB, Malami I, Imam MU, Qusty N, Cruz-Martins N, Batiha GE, Abubakar MB. Uthman YA, et al. Biochem Pharmacol. 2021 Aug;190:114657. doi: 10.1016/j.bcp.2021.114657. Epub 2021 Jun 16. Biochem Pharmacol. 2021. PMID: 34144008 Review. - Targeting lncRNA/Wnt axis by flavonoids: A promising therapeutic approach for colorectal cancer.
Han S, Cao Y, Guo T, Lin Q, Luo F. Han S, et al. Phytother Res. 2022 Nov;36(11):4024-4040. doi: 10.1002/ptr.7550. Epub 2022 Oct 13. Phytother Res. 2022. PMID: 36227024 Review.
Cited by
- Synergistic Mechanisms of Selected Polyphenols in Overcoming Chemoresistance and Enhancing Chemosensitivity in Colorectal Cancer.
Hon KW, Naidu R. Hon KW, et al. Antioxidants (Basel). 2024 Jul 7;13(7):815. doi: 10.3390/antiox13070815. Antioxidants (Basel). 2024. PMID: 39061884 Free PMC article. Review. - Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression.
Ji Q, Liu X, Han Z, Zhou L, Sui H, Yan L, Jiang H, Ren J, Cai J, Li Q. Ji Q, et al. BMC Cancer. 2015 Mar 5;15:97. doi: 10.1186/s12885-015-1119-y. BMC Cancer. 2015. PMID: 25884904 Free PMC article. - Blocking long noncoding RNA MALAT1 restrained the development of laryngeal and hypopharyngeal carcinoma.
Xu E, Liang X, Ji Z, Zhao S, Li L, Lang J. Xu E, et al. Eur Arch Otorhinolaryngol. 2020 Feb;277(2):611-621. doi: 10.1007/s00405-019-05732-x. Epub 2019 Dec 2. Eur Arch Otorhinolaryngol. 2020. PMID: 31792655 Free PMC article. - The function of natural compounds in important anticancer mechanisms.
Nan Y, Su H, Zhou B, Liu S. Nan Y, et al. Front Oncol. 2023 Jan 4;12:1049888. doi: 10.3389/fonc.2022.1049888. eCollection 2022. Front Oncol. 2023. PMID: 36686745 Free PMC article. Review. - LncRNA MALAT1 signaling pathway and clinical applications in overcome on cancers metastasis.
Mazarei M, Shahabi Rabori V, Ghasemi N, Salehi M, Rayatpisheh N, Jahangiri N, Saberiyan M. Mazarei M, et al. Clin Exp Med. 2023 Dec;23(8):4457-4472. doi: 10.1007/s10238-023-01179-x. Epub 2023 Sep 11. Clin Exp Med. 2023. PMID: 37695391 Review.
References
- Fidler IJ (2003) The pathogenesis of cancer metastasis: the seed and soil hypothesis revisited. Nat Rev Cancer 3: 1–6. - PubMed
- Xu TM, Cui MH, Xin Y, Gu LP, Jiang X, et al. (2008) Inhibitory effect of ginsenoside Rg3 on ovarian cancer metastasis. Chin Med J 121: 1394–1397. - PubMed
- Chang JS, Liu HW, Wang KC, Chen MC, Chiang LC, et al. (2005) Ethanol extract of Polygonum cuspidatum inhibits hepatitis B virus in a stable HBV-producing cell line. Antiviral Res 66: 29–34. - PubMed
- Chin YH, Yu PC, Jeli C (2007) Antioxidant activity of extract from Polygonum cuspidatum. Biol Res 40: 13–21. - PubMed
- Feng L, Zhang LF, Yan T, Jin J, Tao WY (2006) Studies on active substance of anticancer effect in Polygonum cuspidatum. Zhong Yao Cai 29: 689–691. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by National Natural Science Foundation of China (81202812, 81303102, 81303103), Program of Shanghai Municipal Education Commission (2011JW57, 12YZ058), Shanghai Municipal Health Bureau (ZYSNXD-CC-YJXYY-JS20, 2011ZJ030, 20114Y001, 20114037), Shanghai Key Laboratory of Traditional Chinese Clinical Medicine (C10dz2220200). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical