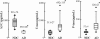CSF Presenilin-1 complexes are increased in Alzheimer's disease - PubMed (original) (raw)
CSF Presenilin-1 complexes are increased in Alzheimer's disease
María-Salud García-Ayllón et al. Acta Neuropathol Commun. 2013.
Abstract
Background: Presenilin-1 (PS1) is the active component of the amyloid precursor protein cleaving γ-secretase complex. PS1 protein is a transmembrane protein containing multiple hydrophobic regions which presence in cerebrospinal fluid (CSF) has not been measured to date. This study assesses whether PS1 and other components of the γ-secretase complex are present in CSF.
Results: Here, we show that PS1 is present in ventricular post-mortem and lumbar ante-mortem CSF, and plasma as 100-150-kDa hetero-complexes containing both the N- and C-terminal fragments (NTF and CTF) of the protein. Immunoprecipitation and immunoblotting with different antibodies confirmed the identity of the PS1 species. The γ-secretase components, APH-1 (anterior pharynx-defective 1) and PEN-2 (presenilin enhancer 2), as well as presenilin-2 (PS2) fragments, co-exist within these CSF complexes, while nicastrin is not detected. These CSF-PS1 complexes differ from active γ-secretase membrane-complexes, and may represent nonspecific aggregation of the PS1 protein. Levels of PS1 complexes are increased in CSF samples from autopsy-confirmed Alzheimer's disease (AD) cases and were found to be more stable than complexes in CSF from control subjects. Despite similar levels of total PS1 in CSF from probable AD patients and cognitively normal subjects, an increased proportion of highly stable PS1 complexes were observed in AD CSF.
Conclusions: Our data suggest that fragments of the PS1 protein present in CSF as complexes may be useful as a biomarker for AD.
Figures
Figure 1
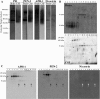
PS1 complexes are detected in human CSF with alternative antibodies. (A) Schematic representation of PS1 with epitopes for the anti-PS1 antibodies indicated. (B) Human ventricular post-mortem CSF samples from non-demented controls (NDC) were blotted under denaturing conditions with the indicated anti-PS1 antibodies. (C) Loss of CSF-PS1 reactivity in CSF samples heated at 98°C compared to 50°C using the NTF-PS1 antibody from Calbiochem. The stability of CSF-PS1 is also affected after 20 minutes of heating at 50°C resulting in loss of immunoreactivity. (D) CSF samples were also blotted for the homologue PS2 with an anti-CTF antibody (00/12) and for other subunits of the γ-secretase complexes, APH-1, PEN-2 and nicastrin. Nicastrin was the only γ-secretase partner with a different banding pattern, with a 130-kDa band corresponding to the molecular mass of the protein, which was weakly detectable in some of the CSF samples tested. The ~35-kDa band (*) may correspond to nonspecific binding by the antibody, as fragments of this size have not been previously demonstrated to be positive for nicastrin. (E) Effect of denaturation temperature prior to electrophoresis (heating for 5 min at 98°C or 10 min at 50°C) on stability of APH-1, PEN-2 and nicastrin. High molecular mass complexes of APH-1 and PEN-2 were only detected after heating at 50°C, while the 130 kDa-kDa nicastrin subunit was detected after denaturation at 98°C, indicating that nicastrin is not a part of the complexes. Illustrative examples (from 3 different experiments).
Figure 2
Immunoprecipitation of PS1 in human CSF. (A) Ventricular post-mortem CSF was immunoprecipitated with the anti-PS1 98/1 antibody, and precipitated proteins (IP) were immunoblotted with PS1-NTF (Calbiochem) or PS1-CTF antibodies (00/2). Precipitated proteins (IP) were immunoblotted with the PS2-CTF antibody 00/12. (B) Lumbar ante-mortem CSF was immunoprecipitated with NTF-PS1 antibody 98/1 or with antibody from Thermo Scientific, and precipitated proteins (IP) detected with an NTF-PS1 antibody from Calbiochem. (C) Extracts from SH-SY5Y cells over-expressing PS1 (+) and extracted in the presence of 1% Nonidet P-40/0.5% Triton X-100 were run in parallel with post mortem CSF samples from non-diseased control subjects. The presence of a ~43 kDa PS1 holoprotein in membrane extracts was detected with the NTF-PS1 antibody from Calbiochem. Comparison of the immunoreactive bands indicates that this 50-kDa band is not the PS1 holoprotein. (D) PS1-precipitated proteins (PS1-IP) were also immunoblotted with anti APH-1, PEN-2 or nicastrin antibodies. APH-1 and PEN-2, but not nicastrin were detected in PS1 immunoprecipitates. Extracts incubated with protein A-Sepharose with an irrelevant rabbit IgG (Bound IgG), were analyzed in parallel as negative controls.
Figure 3
Characterization of CSF-PS1 complexes. (A) Brain γ-secretase complexes (frontal cortex from non-diseased control subjects extracted in buffer containing 0.5% dodecylmaltoside) were analyzed by blue native-PAGE and compared with PS1 complexes isolated from lumbar CSF samples (NDC cases). Incubation of blots with antibodies for the different γ-secretase subunits confirmed that nicastrin is not present in CSF-PS1 complexes. (B) The same brain extract and CSF samples were also fractionated on 5-20% sucrose density gradients. The fractions (collected from the top of each tube) were immunoblotted for NTF-PS1 under denaturing conditions with the antibody from Calbiochem. Enzymes of known sedimentation coefficient, β-galactosidase (G, 16.0S; ~540 kDa), catalase (C, 11.4S; ~232 kDa) and alkaline phosphatase (P, 6.1S; ~140-160 kDa) were used as internal markers. (C) CSF fractions from sucrose gradients were also blotted for APH-1, PEN-2 and nicastrin. Nicastrin was mostly undetectable in fractionated CSF samples.
Figure 4
PS1 complexes are present in human and mouse CSF and plasma. (A) Plasma samples from NDC subjects were blotted with an anti-NTF-PS1 antibody (from Calbiochem) with and without depletion of plasma abundant proteins by immunoaffinity-based protein subtraction chromatography with IgY microbeads (Seppro™). (B) CSF and plasma samples from control wild-type (WT) and PS1 conditional knockout mice (cKO) were blotted with an anti-NTF-PS1 antibody. In general, immunoreactivities of the bands corresponding to PS1 complexes were weak in mouse (or rat, not shown) than in human samples. PS1 levels are reduced in CSF from PS1 cKO mice, and unchanged in plasma. PS1 immunoreactivity detected in CSF from PS1 cKO mice may be due to PS1 expressed in glia and interneurons. However, it is also highly likely that some nonspecific binding may occur at 50 kDa (*).
Figure 5
PS1 stable complexes are increased in AD post-mortem CSF. (A) Representative blot of NTF-PS1 in post-mortem CSF samples from 10 AD (open circles) and 7 NDC controls (closed circles). The densitometric quantification of the accumulative immunoreactivity from the sum of the higher molecular mass PS1 complex (100 + 150 kDa) is shown. (B) Immunodetection and densitometric quantification of higher molecular mass PS1 complex (100 + 150 kDa) from CSF samples blotted with an anti-CTF-PS1 antibody. Dashed lines represent arbitrary cutoffs that maximally discriminated between AD and NDC groups. (C) PS1 complexes in 4 (of 7) NDC (closed circles) and 8 (of 10) AD CSF samples (open circles) were fractionated on 5-20% sucrose density gradients. The fractions (collected from the top of each tube) were immunoblotted for NTF-PS1 under denaturing conditions with the antibody from Calbiochem. Enzymes of known sedimentation coefficient, β-galactosidase (G, 16.0S; ~540 kDa), catalase (C, 11.4S; ~232 kDa) and alkaline phosphatase (P, 6.1S; ~140-160 kDa) were used as internal markers. Representative blots are shown. A quotient between highly stable complexes (100 + 150 kDa immunoreactive bands sediment closer to alkaline phosphatase, fractions 2-7) and unstable complexes (50-kDa immunoreactive bands sediment closer to catalase, fractions 8-12) was defined and represented. Dashed lines represent an arbitrary cutoff that maximally discriminated between AD and NDC groups. The data represent the means (in arbitrary units) ± SEM. *Significantly different (p < 0.05) from the NDC group, as assessed by the Mann-Whitney U test.
Figure 6
Levels of classical AD biomarkers in lumbar CSF. Box plot of CSF levels of Aβ42, T-tau and P-tau for the 12 probable AD cases (open circles) and the 12 NDC controls (closed circles). Means ± SEM are shown. Mann-Whitney U test, *p < 0.05, **p < 0.01.
Figure 7
Stable PS1 complexes are increased in AD ante-mortem CSF. (A) Representative blot and densitometric quantification of the accumulative immunoreactivity from the sum of higher molecular mass PS1 complex (100 + 150 kDa) in lumbar CSF samples from 12 AD (open circles) and 12 NDC controls (closed circles). (B) Eight of the 12 cases available from both the AD and NDC groups were also fractioned into 5-20% sucrose density gradients, and blotted with the NTF-PS1 antibody under denaturing conditions. Internal markers were β-galactosidase (G), catalase (C) and alkaline phosphatase (P), as in Figure 5. In the right panel the individual values for the quotient between highly stable complexes (100 + 150 kDa immunoreactive bands sediment at fractions 2-7) and unstable complexes (50 kDa immunoreactive bands sediment at fractions 8-12) are shown. *Significantly different (p < 0.05) from the NDC group, as assessed by Mann-Whitney U test.
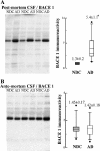
Figure 8
BACE1 levels in the post-mortem and ante-mortem CSF samples. (A) Immunodetection and densitometric quantification of the ~70-kDa immunoreactive BACE1 band from the same post-mortem CSF cases, AD (open circles) and NDC (closed circles), presented in Figure 5; and (B) from the same ante-mortem CSF cases, AD (open circles) and NDC (closed circles), presented in Figure 6. The box blot of BACE1 levels includes the means of the immunoreactivities (in arbitrary units) ± SEM (determinations by duplicate). *p < 0.05 significantly different from NDC group, as assessed by the Student’s t test.
Similar articles
- gamma-Secretase complexes containing N- and C-terminal fragments of different presenilin origin retain normal gamma-secretase activity.
Strömberg K, Hansson EM, Laudon H, Bergstedt S, Näslund J, Lundkvist J, Lendahl U. Strömberg K, et al. J Neurochem. 2005 Nov;95(3):880-90. doi: 10.1111/j.1471-4159.2005.03415.x. Epub 2005 Aug 31. J Neurochem. 2005. PMID: 16135086 - Nicastrin is critical for stability and trafficking but not association of other presenilin/gamma-secretase components.
Zhang YW, Luo WJ, Wang H, Lin P, Vetrivel KS, Liao F, Li F, Wong PC, Farquhar MG, Thinakaran G, Xu H. Zhang YW, et al. J Biol Chem. 2005 Apr 29;280(17):17020-6. doi: 10.1074/jbc.M409467200. Epub 2005 Feb 11. J Biol Chem. 2005. PMID: 15711015 Free PMC article. - Nicastrin, presenilin, APH-1, and PEN-2 form active gamma-secretase complexes in mitochondria.
Hansson CA, Frykman S, Farmery MR, Tjernberg LO, Nilsberth C, Pursglove SE, Ito A, Winblad B, Cowburn RF, Thyberg J, Ankarcrona M. Hansson CA, et al. J Biol Chem. 2004 Dec 3;279(49):51654-60. doi: 10.1074/jbc.M404500200. Epub 2004 Sep 28. J Biol Chem. 2004. PMID: 15456764 - From presenilinase to gamma-secretase, cleave to capacitate.
Xia W. Xia W. Curr Alzheimer Res. 2008 Apr;5(2):172-8. doi: 10.2174/156720508783954712. Curr Alzheimer Res. 2008. PMID: 18393802 Review. - Genes and mechanisms involved in beta-amyloid generation and Alzheimer's disease.
Steiner H, Capell A, Leimer U, Haass C. Steiner H, et al. Eur Arch Psychiatry Clin Neurosci. 1999;249(6):266-70. doi: 10.1007/s004060050098. Eur Arch Psychiatry Clin Neurosci. 1999. PMID: 10653281 Review.
Cited by
- Heteromers of amyloid precursor protein in cerebrospinal fluid.
Cuchillo-Ibañez I, Lopez-Font I, Boix-Amorós A, Brinkmalm G, Blennow K, Molinuevo JL, Sáez-Valero J. Cuchillo-Ibañez I, et al. Mol Neurodegener. 2015 Jan 8;10:2. doi: 10.1186/1750-1326-10-2. Mol Neurodegener. 2015. PMID: 25573162 Free PMC article. - Is liquid biopsy mature enough for the diagnosis of Alzheimer's disease?
Gong X, Zhang H, Liu X, Liu Y, Liu J, Fapohunda FO, Lü P, Wang K, Tang M. Gong X, et al. Front Aging Neurosci. 2022 Aug 5;14:977999. doi: 10.3389/fnagi.2022.977999. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35992602 Free PMC article. Review. - Dendrobium alkaloids decrease Aβ by regulating α- and β-secretases in hippocampal neurons of SD rats.
Huang J, Huang N, Zhang M, Nie J, Xu Y, Wu Q, Shi J. Huang J, et al. PeerJ. 2019 Sep 6;7:e7627. doi: 10.7717/peerj.7627. eCollection 2019. PeerJ. 2019. PMID: 31534855 Free PMC article. - The Crocus sativus Compounds _trans_-Crocin 4 and _trans_-Crocetin Modulate the Amyloidogenic Pathway and Tau Misprocessing in Alzheimer Disease Neuronal Cell Culture Models.
Chalatsa I, Arvanitis DA, Koulakiotis NS, Giagini A, Skaltsounis AL, Papadopoulou-Daifoti Z, Tsarbopoulos A, Sanoudou D. Chalatsa I, et al. Front Neurosci. 2019 Mar 26;13:249. doi: 10.3389/fnins.2019.00249. eCollection 2019. Front Neurosci. 2019. PMID: 30971876 Free PMC article. - Altered Balance of Reelin Proteolytic Fragments in the Cerebrospinal Fluid of Alzheimer's Disease Patients.
Lopez-Font I, Lennol MP, Iborra-Lazaro G, Zetterberg H, Blennow K, Sáez-Valero J. Lopez-Font I, et al. Int J Mol Sci. 2022 Jul 7;23(14):7522. doi: 10.3390/ijms23147522. Int J Mol Sci. 2022. PMID: 35886870 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical