Computational models reveal a passive mechanism for cell migration in the crypt - PubMed (original) (raw)
Computational models reveal a passive mechanism for cell migration in the crypt
Sara-Jane Dunn et al. PLoS One. 2013.
Abstract
Cell migration in the intestinal crypt is essential for the regular renewal of the epithelium, and the continued upward movement of cells is a key characteristic of healthy crypt dynamics. However, the driving force behind this migration is unknown. Possibilities include mitotic pressure, active movement driven by motility cues, or negative pressure arising from cell loss at the crypt collar. It is possible that a combination of factors together coordinate migration. Here, three different computational models are used to provide insight into the mechanisms that underpin cell movement in the crypt, by examining the consequence of eliminating cell division on cell movement. Computational simulations agree with existing experimental results, confirming that migration can continue in the absence of mitosis. Importantly, however, simulations allow us to infer mechanisms that are sufficient to generate cell movement, which is not possible through experimental observation alone. The results produced by the three models agree and suggest that cell loss due to apoptosis and extrusion at the crypt collar relieves cell compression below, allowing cells to expand and move upwards. This finding suggests that future experiments should focus on the role of apoptosis and cell extrusion in controlling cell migration in the crypt.
Conflict of interest statement
Competing Interests: We can confirm that two of the authors are affiliated with Microsoft Research – one by direct employment and the second as a visiting researcher. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials.
Figures
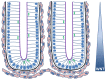
Figure 1. A cartoon sketch illustrating two neighbouring crypts.
The nuclei of the epithelial cells are indicated in blue, and the arrows illustrate the typical alignment of the mitotic spindle during division for various cell positions. The apical surface of each epithelial cell faces the crypt lumen (purple) while the basal surface is in contact with the basement membrane (black). The myofibroblasts that form the pericryptal fibroblast sheath are coloured pink. A decreasing gradient of Wnt signalling factors exists along the crypt axis, influencing the proliferative state of the epithelial cells.
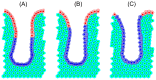
Figure 2. Simulation snapshots for each crypt model at steady state.
Proliferative cells are coloured yellow, differentiated cells are coloured pink, and in (C), stromal cells are coloured green.
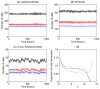
Figure 3. Epithelial cell number at steady state for each of the computational models.
(A) - (C) Comparison of epithelial cell number at steady state for each model: the total number of epithelial cells (black) and subdivided into the number of proliferative (red) and non-proliferative cells (blue). (D) The average number of cells within bands of width 1 cell diameter for the 2D cylindrical model (solid line) and 3D rigid model (dashed line), and cross-sectional model (dotted line).
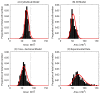
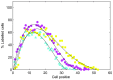
Figure 4. Epithelial cell size for each of the computational models.
(A) - (C) The epithelial cell area distribution for each model – the red curve marks the fitted Gamma distribution in each case. The vertical dotted line marks the equilibrium area (86.6 μm2), and the proportion of the total number of epithelial cells is indicated on the y-axis. (D) Experimental data obtained from three wildtype murine tissue samples, taken from midway down the length of the colon. ((C) and (D) re-plotted from data provided in [34]).
Figure 5. Average epithelial cell velocity, vy, along the longitudinal crypt axis for each model.
2D cylindrical model (solid line), 3D rigid model (dashed line), cross-sectional model (dotted line), experimental data from [41] (dot-dashed line). The vertical dotted line marks the highest point of the proliferative compartment.
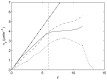
Figure 6. An example of experimental results showing labelled cell distributions at specific time intervals following irradiation.
% Labelling index corresponds to the percentage of labelled cells in the crypt. (●) 9 hours, (▲) 12 hours, (■) 24 hours. Data re-plotted from [41].
Figure 7. Snapshots of the cylindrical crypt model following elimination of cell division.
(A) At 0 hours; the blue cells are those which were originally proliferative but are now labelled, (B) 12 hours later, (C) 24 hours later.
Figure 8. Snapshots of the 3D crypt model following elimination of cell division.
(A) At 0 hours, (B) 12 hours later, (C) 24 hours later.
Figure 9. Snapshots of the 2D cross-sectional crypt model following elimination of cell division.
(A) At 0 hours, (B) 12 hours later, (C) 24 hours later. These snapshots highlight that the crypt becomes shorter over time.
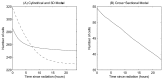
Figure 10. The change in epithelial cell number following elimination of cell proliferation.
(A) Comparing the 2D cylindrical model (solid line) and the 3D rigid model (dashed line), (B) the cross-sectional model.
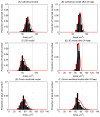
Figure 11. The change in the distribution of cell sizes following elimination of cell division.
(A) and (B) correspond to the cylindrical model; (C) and (D) correspond to the 3D model; (E) and (F) correspond to the cross-sectional model. The left column indicates the distribution of cell areas for the model at steady state, and the right column shows the distribution of cell areas 24 hours post irradiation. The red curves mark the fitted Gamma distribution. The equilibrium cell are (86.6 μm2) is indicated by the vertical dotted line on each plot.
Figure 12. The change in the distribution of labelled cells following the elimination of cell division.
Each curve corresponds to the percentage of labelled cells within horizontal bands of width 1 cell diameters after 1 hour, 3 hours, 6 hours, 9 hours, 12 hours and 24 hours (averaged over 50 simulations in each case). The arrow indicates the movement of the distribution with increasing time. Plot (D) shows the results found by Kaur and Potten for 9, 12 and 24 hours, edited from Figure 6(B) to facilitate comparison with in silico results.
Similar articles
- Cell migration and organization in the intestinal crypt using a lattice-free model.
Meineke FA, Potten CS, Loeffler M. Meineke FA, et al. Cell Prolif. 2001 Aug;34(4):253-66. doi: 10.1046/j.0960-7722.2001.00216.x. Cell Prolif. 2001. PMID: 11529883 Free PMC article. - An individual based computational model of intestinal crypt fission and its application to predicting unrestrictive growth of the intestinal epithelium.
Pin C, Parker A, Gunning AP, Ohta Y, Johnson IT, Carding SR, Sato T. Pin C, et al. Integr Biol (Camb). 2015 Feb;7(2):213-28. doi: 10.1039/c4ib00236a. Integr Biol (Camb). 2015. PMID: 25537618 - Computational model of cell positioning: directed and collective migration in the intestinal crypt epithelium.
Wong SY, Chiam KH, Lim CT, Matsudaira P. Wong SY, et al. J R Soc Interface. 2010 Jun 6;7 Suppl 3(Suppl 3):S351-63. doi: 10.1098/rsif.2010.0018.focus. Epub 2010 Mar 31. J R Soc Interface. 2010. PMID: 20356873 Free PMC article. - Human cell models to study small intestinal functions: recapitulation of the crypt-villus axis.
Pageot LP, Perreault N, Basora N, Francoeur C, Magny P, Beaulieu JF. Pageot LP, et al. Microsc Res Tech. 2000 May 15;49(4):394-406. doi: 10.1002/(SICI)1097-0029(20000515)49:4<394::AID-JEMT8>3.0.CO;2-K. Microsc Res Tech. 2000. PMID: 10820523 Review. - Stem cell self-renewal in intestinal crypt.
Simons BD, Clevers H. Simons BD, et al. Exp Cell Res. 2011 Nov 15;317(19):2719-24. doi: 10.1016/j.yexcr.2011.07.010. Epub 2011 Jul 20. Exp Cell Res. 2011. PMID: 21787769 Review.
Cited by
- Enabling Anyone to Translate Clinically Relevant Ideas to Therapies.
Ekins S, Diaz N, Chung J, Mathews P, McMurtray A. Ekins S, et al. Pharm Res. 2017 Jan;34(1):1-6. doi: 10.1007/s11095-016-2039-5. Epub 2016 Sep 12. Pharm Res. 2017. PMID: 27620174 - Regulators of Intestinal Epithelial Migration in Sepsis.
Meng M, Klingensmith NJ, Liang Z, Lyons JD, Fay KT, Chen CW, Ford ML, Coopersmith CM. Meng M, et al. Shock. 2019 Jan;51(1):88-96. doi: 10.1097/SHK.0000000000001117. Shock. 2019. PMID: 29424793 Free PMC article. - Truncated Adenomatous Polyposis Coli Mutation Induces Asef-Activated Golgi Fragmentation.
Kim SB, Zhang L, Yoon J, Lee J, Min J, Li W, Grishin NV, Moon YA, Wright WE, Shay JW. Kim SB, et al. Mol Cell Biol. 2018 Aug 15;38(17):e00135-18. doi: 10.1128/MCB.00135-18. Print 2018 Sep 1. Mol Cell Biol. 2018. PMID: 29866653 Free PMC article. - Multiscale Model of Colorectal Cancer Using the Cellular Potts Framework.
Osborne JM. Osborne JM. Cancer Inform. 2015 Oct 4;14(Suppl 4):83-93. doi: 10.4137/CIN.S19332. eCollection 2015. Cancer Inform. 2015. PMID: 26461973 Free PMC article. - The role of cell location and spatial gradients in the evolutionary dynamics of colon and intestinal crypts.
Shahriyari L, Komarova NL, Jilkine A. Shahriyari L, et al. Biol Direct. 2016 Aug 23;11(1):42. doi: 10.1186/s13062-016-0141-6. Biol Direct. 2016. PMID: 27549762 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources