Exendin-4 ameliorates traumatic brain injury-induced cognitive impairment in rats - PubMed (original) (raw)
Exendin-4 ameliorates traumatic brain injury-induced cognitive impairment in rats
Katharine Eakin et al. PLoS One. 2013.
Abstract
Traumatic brain injury represents a major public health issue that affects 1.7 million Americans each year and is a primary contributing factor (30.5%) of all injury-related deaths in the United States. The occurrence of traumatic brain injury is likely underestimated and thus has been termed "a silent epidemic". Exendin-4 is a long-acting glucagon-like peptide-1 receptor agonist approved for the treatment of type 2 diabetes mellitus that not only effectively induces glucose-dependent insulin secretion to regulate blood glucose levels but also reduces apoptotic cell death of pancreatic β-cells. Accumulating evidence also supports a neurotrophic and neuroprotective role of glucagon-like peptide-1 in an array of cellular and animal neurodegeneration models. In this study, we evaluated the neuroprotective effects of Exendin-4 using a glutamate toxicity model in vitro and fluid percussion injury in vivo. We found neuroprotective effects of Exendin-4 both in vitro, using markers of cell death, and in vivo, using markers of cognitive function, as assessed by Morris Water Maze. In combination with the reported benefits of ex-4 in other TBI models, these data support repositioning of Exendin-4 as a potential treatment for traumatic brain injury.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
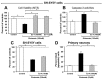
Figure 1. Studies of Exendin-4 in vitro.
Activation of GLP-1R signaling by Exendin-4 (Ex-4) protects both human SH-SY5Y cells and rat primary neurons from glutamate-induced excitotoxicity. (A) The cellular viability of SH-SY5Y cells challenged with glutamate (100 mM) was significantly reduced (37.5%) following 24 hr incubation, as assessed by MTS assay. Pretreatment with Ex-4 (100 nM) fully mitigated this glutamate-induced cellular loss (p<0.05 vs. glutamate alone challenged group, Dunnett’s _t_-test, N≥3 per treatment group). (B) In line with glutamate-induced excitotoxic cell loss, caspase-3 levels were significantly elevated in SH-SY5Y cells. Pretreatment with Exendin-4 fully ameliorated this rise (p<0.05 vs. glutamate alone challenged group, Dunnett’s T test, N≥3 per treatment group). (C) Co-treatment of SH-SY5Y cells with glutamate (100 mM) and concentrations as low as 10 nM Ex-4 resulted in significant mitigation of excitotoxicity, as assessed by MTS assay at 24 hr (p<0.05 vs. glutamate alone challenged group, Dunnett’s _t_-test, N≥3 per treatment group). (D) In parallel studies, rat primary neurons proved vulnerable to glutamate challenge (10 μM) which induced substantial apoptosis (73.1%). Pretreatment with Ex-4 (300 nM) significantly mitigated this, lowering levels to those of unchallenged control neuronal cultures (p<0.05 vs. glutamate alone challenged group, Dunnett’s _t_-test, N≥3 per treatment group). The glutamate challenge concentrations (100 mM for SH-SY5Y cells and 10 μM for primary neurons) and time line were selected from preliminary studies focused on inducing a significant yet incomplete cellular loss to allow for assessment of mitigation as well as a potential intensification of cell death by the experimental treatment.
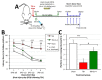
Figure 2. Studies of Exendin-4 in vivo.
Administration of Exendin-4 (Ex-4) significantly ameliorated cognitive impairments induced by fluid percussion-induced TBI in rats assessed by the Morris Water Maze (MWM) paradigm. (A) Time line of study: 30 min following fluid percussion injury-induced TBI or sham treatment, rats were administered either saline or Ex-4 for 7 days (initially, a loading dose of Ex-4 was administered followed by steady-state subcutaneous (s.c.) infusion via an implanted Alzet mini pump). Thereafter on days 10 to 14, animals were subjected to MWM assessment. (B). Post-injury administration of Ex-4 significantly improved MWM performance. Cognitive performance as assessed by latency to locate the hidden platform in the MWM was compared for each of the three treatment groups, TBI (n = 9), TBI+Ex-4 (n = 8), sham (n = 9). A repeated-measures ANOVA showed significant between group differences. Post hoc analysis using Fishers LSD revealed that animals that were treated with Ex-4 after TBI had significantly shorter latencies to reach the goal platform as compared to injured saline-treated. No significant difference was observed in the performance between TBI+Ex-4 and sham groups. Data are presented as the mean ± SEM. ***_p_≤ .001, sham relative to TBI. †† _p_≤ .01, Ex-4 relative to TBI. (C) Number of platform zone crossings during the probe trial. Cumulative learning for the platform location was assessed during the probe trial as the number of times the animals swam within a 7.5 cm radius of the platform border (i.e., 2x the diameter of the platform). One-way ANOVA indicated significant between group differences. Fisher’s LSD post hoc showed that sham and TBI+Ex-4 had better retention of the platform location relative to animals that received TBI only. Data are presented as the mean ± SEM. Brackets indicate direct comparisons between groups. *_p_≤ .05, ***p ≤ .001.
Similar articles
- Liraglutide is neurotrophic and neuroprotective in neuronal cultures and mitigates mild traumatic brain injury in mice.
Li Y, Bader M, Tamargo I, Rubovitch V, Tweedie D, Pick CG, Greig NH. Li Y, et al. J Neurochem. 2015 Dec;135(6):1203-1217. doi: 10.1111/jnc.13169. Epub 2015 Jun 18. J Neurochem. 2015. PMID: 25982185 Free PMC article. - Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice.
Rachmany L, Tweedie D, Li Y, Rubovitch V, Holloway HW, Miller J, Hoffer BJ, Greig NH, Pick CG. Rachmany L, et al. Age (Dordr). 2013 Oct;35(5):1621-36. doi: 10.1007/s11357-012-9464-0. Epub 2012 Aug 15. Age (Dordr). 2013. PMID: 22892942 Free PMC article. - Glucagon-like peptide-1 receptor agonist Exendin-4 improves neurological outcomes by attenuating TBI- induced inflammatory responses and MAPK activation in rats.
Zhang J, Yi T, Cheng S, Zhang S. Zhang J, et al. Int Immunopharmacol. 2020 Sep;86:106715. doi: 10.1016/j.intimp.2020.106715. Epub 2020 Jun 20. Int Immunopharmacol. 2020. PMID: 32570036 - Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes.
Nielsen LL, Young AA, Parkes DG. Nielsen LL, et al. Regul Pept. 2004 Feb 15;117(2):77-88. doi: 10.1016/j.regpep.2003.10.028. Regul Pept. 2004. PMID: 14700743 Review. - Is exenatide advancing the treatment of type 2 diabetes?
Doggrell SA. Doggrell SA. Expert Opin Pharmacother. 2006 Jan;7(1):109-12. doi: 10.1517/14656566.7.1.109. Expert Opin Pharmacother. 2006. PMID: 16370928 Review.
Cited by
- Preservation of the blood brain barrier and cortical neuronal tissue by liraglutide, a long acting glucagon-like-1 analogue, after experimental traumatic brain injury.
Hakon J, Ruscher K, Romner B, Tomasevic G. Hakon J, et al. PLoS One. 2015 Mar 30;10(3):e0120074. doi: 10.1371/journal.pone.0120074. eCollection 2015. PLoS One. 2015. PMID: 25822252 Free PMC article. - Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: Implications for neurodegenerative disease treatment.
Kopp KO, Glotfelty EJ, Li Y, Greig NH. Kopp KO, et al. Pharmacol Res. 2022 Dec;186:106550. doi: 10.1016/j.phrs.2022.106550. Epub 2022 Nov 11. Pharmacol Res. 2022. PMID: 36372278 Free PMC article. Review. - L-Carnitine and extendin-4 improve outcomes following moderate brain contusion injury.
Chen H, Chan YL, Linnane C, Mao Y, Anwer AG, Sapkota A, Annissa TF, Herok G, Vissel B, Oliver BG, Saad S, Gorrie CA. Chen H, et al. Sci Rep. 2018 Jul 25;8(1):11201. doi: 10.1038/s41598-018-29430-6. Sci Rep. 2018. PMID: 30046063 Free PMC article. - Making sense of gut feelings in the traumatic brain injury pathogenesis.
Royes LFF, Gomez-Pinilla F. Royes LFF, et al. Neurosci Biobehav Rev. 2019 Jul;102:345-361. doi: 10.1016/j.neubiorev.2019.05.012. Epub 2019 May 16. Neurosci Biobehav Rev. 2019. PMID: 31102601 Free PMC article. Review. - The Emerging Role of GLP-1 Receptors in DNA Repair: Implications in Neurological Disorders.
Yang JL, Chen WY, Chen SD. Yang JL, et al. Int J Mol Sci. 2017 Aug 26;18(9):1861. doi: 10.3390/ijms18091861. Int J Mol Sci. 2017. PMID: 28846606 Free PMC article. Review.
References
- Faul MD, Xu L, Wald M, Coronado V (2010) Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; pp. 2002-2006.
- Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM et al. (2011) Centers for Disease Control and Prevention (CDC). Surveillance for traumatic brain injury-related deaths--United States, 1997-2007. MMWR Surveill Summ 6:60(5): 1-32. - PubMed
- CDC (2010) Injury, prevention, & control: traumatic brain injury. Center for Disease Control and Prevention. Available online at: http://www.cdc.gov/traumaticbraininjury/statistics.html.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources