Mad1 kinetochore recruitment by Mps1-mediated phosphorylation of Bub1 signals the spindle checkpoint - PubMed (original) (raw)
Mad1 kinetochore recruitment by Mps1-mediated phosphorylation of Bub1 signals the spindle checkpoint
Nitobe London et al. Genes Dev. 2014.
Abstract
The spindle checkpoint is a conserved signaling pathway that ensures genomic integrity by preventing cell division when chromosomes are not correctly attached to the spindle. Checkpoint activation depends on the hierarchical recruitment of checkpoint proteins to generate a catalytic platform at the kinetochore. Although Mad1 kinetochore localization is the key regulatory downstream event in this cascade, its receptor and mechanism of recruitment have not been conclusively identified. Here, we demonstrate that Mad1 kinetochore association in budding yeast is mediated by phosphorylation of a region within the Bub1 checkpoint protein by the conserved protein kinase Mps1. Tethering this region of Bub1 to kinetochores bypasses the checkpoint requirement for Mps1-mediated kinetochore recruitment of upstream checkpoint proteins. The Mad1 interaction with Bub1 and kinetochores can be reconstituted in the presence of Mps1 and Mad2. Together, this work reveals a critical mechanism that determines kinetochore activation of the spindle checkpoint.
Keywords: Bub1 kinase; Mad1; Mps1 kinase; kinetochore; spindle checkpoint.
Figures
Figure 1.
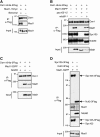
Mad1 associates with purified kinetochores and requires Spc105. (A) Mad1 associates with kinetochore particles when cells are treated with benomyl. A Dsn1-6His-3Flag Mad1-13myc strain (SBY8412) was grown with or without benomyl for 2 h before harvest. Kinetochore particles were purified by α-Flag immunoprecipitation and analyzed by immunoblotting with α-Flag and α-myc antibodies. (B) Spc105 is required for Mad1 kinetochore association. Kinetochore particles were purified by immunoprecipitation of Dsn1-6His-3Flag from cells shifted to 37°C before harvest. Strains contain Dsn1-6His-3Flag and either untagged Mad1 (SBY8253), Mad1-3GFP (SBY8415), Mad1-3GFP spc105-15 (SBY8829), or Mad1-3GFP ndc80-1 (SBY8579). Immunoblots were probed with α-Flag, α-GFP, α-Spc105, and α-Ndc80 antibodies. Note that Ndc80-1 is a truncation and still associates with purified kinetochores. (C) Mad1-3GFP enrichment with Dsn1-6His-3Flag does not require a fully functional spindle checkpoint. Kinetochore particles were purified from the following strains after treatment with benomyl: untagged Mad1 (SBY8253), Mad1-3GFP (SBY8415), or Mad1-3GFP mad3Δ (SBY11497). Immunoblots were probed with α-Flag or α-GFP antibodies. (D) Mad1 copurifies with Spc105c but not with Ndc80c. α-Flag immunoprecipitations of Spc105-3Flag (SBY8970) or Nuf2-3Flag (SBY8971) from benomyl-treated cells containing Mad1-3GFP. Immunoblots were probed with α-Flag, α-GFP, α-Ndc80, or α-Spc105 antibodies. Arrows indicate the migration of Spc105-3Flag or Nuf2-3Flag or the expected migration of untagged endogenous Spc105 (shown at the bottom). Migration of molecular weight markers (in kilodaltons) is shown to the left of each blot. Approximate estimated molecular weights are indicated in parentheses where molecular weight markers were not present within the cropped region.
Figure 2.
Mad1-3A and Bub1ΔM prevent Mad1 kinetochore association and checkpoint function. (A) The Mad1-3A mutant protein does not associate with kinetochores. Kinetochore particles were purified from cells containing untagged Mad1 (SBY8253), Mad1-9myc (SBY11840), or Mad1-3A-9myc (SBY11967). Immunoblots were probed with α-Flag or α-myc antibodies. (B, top) Bub1 schematic showing the N-terminal (1–368) and middle (369–608) regions and C-terminal kinase (609–1021) domain. The N-terminal region contains the conserved tetratricopeptide repeat (TPR) domain (residues 57–171) (Bolanos-Garcia et al. 2009) as well as the Bub3-binding/GLEBS domain (residues 315–350) (Larsen et al. 2007). Conserved domain 1 (CD1; residues 452–470 (Klebig et al. 2009), is present in the middle region. (Bottom) The middle region is deleted in Bub1ΔM. (C) The Bub1 middle region is required for Mad1 kinetochore association. Kinetochore particles were purified via Dsn1-6His-3Flag from cells with untagged Mad1 (SBY8253), Mad1-3GFP (SBY11392), Mad1-3GFP Bub1-9myc (SBY11317), or Mad1-3GFP Bub1ΔM-9myc (SBY11319). Immunoblots were probed with α-Flag, α-myc, or α-GFP antibodies. (D) bub1ΔM cells are benomyl-sensitive. Fivefold serial dilutions of the following strains were plated onto YPD or YPD + 5 μg/mL benomyl: wild type (WT) (SBY8412), mad1Δ (SBY8820), bub1Δ (SBY10596), bub1Δ + integrated 9myc control vector (SBY11232), bub1Δ + integrated BUB1-9myc (SBY11072), and bub1Δ + integrated bub1ΔM-9myc (SBY11083). Note that the cells for the immunoprecipitation experiments shown in this figure were treated with benomyl.
Figure 3.
Bub1(M) mediates Mad1 kinetochore association and checkpoint function. (A) Bub1(M) is sufficient to recruit Mad1 to Spc105-6A mutant kinetochores. Kinetochore particles were purified by α-Flag immunoprecipitation of Dsn1-6His-3Flag from cells with endogenous Mad1 and Spc105-9myc (SBY10267) or cells with Mad1-3GFP and either Spc105-9myc (SBY11207), Bub1(M)-Spc105-9myc (SBY11208), Spc105-6A-9myc (SBY11210), or Bub1(M)-Spc105-6A-9myc (SBY11209). Immunoblots were probed with α-Flag or α-GFP antibodies. (B) Bub1(M)-Spc105-6A mutant kinetochores do not recruit endogenous Bub1/3. Kinetochores were purified as in A from the following strains: Spc105-9myc (SBY10267), Bub1-3GFP Spc105-9myc (SBY10347), Bub1-3GFP Spc105-6A-9myc (SBY10422), Bub1-3GFP Bub1(M)-Spc105-6A-9myc (SBY11725), Bub3-3GFP Spc105-9myc (SBY10351), Bub3-3GFP Spc105-6A-9myc (SBY10372), and Bub3-3GFP Bub1(M)-Spc105-6A-9myc (SBY11637). Immunoblots were probed with α-Flag or α-GFP antibodies. (C) Bub1(M) is sufficient to recruit Mad1 to Spc105-6A mutant kinetochores in the absence of endogenous Bub1. Kinetochore particles were purified as in A from strains containing Mad1-3GFP with Spc105-9myc (SBY11207), Bub1(M)-Spc105-6A-9myc (SBY11209), Spc105-6A-9myc (SBY11210), or Bub1(M)-Spc105-6A-9myc bub1Δ (SBY11303). Immunoblots were probed with α-Flag or α-GFP antibodies. (D) The middle region of Bub1 can restore partial checkpoint function in spc105-6A mutant cells. Cells were released from G1 into nocodazole-containing medium. Samples were taken at the indicated time points (in minutes) to analyze Pds1-18myc levels by immunoblotting with α-myc antibodies in SPC105-2V5 (SBY11892), spc105-6A-2V5 (SBY11893), and bub1(M)-spc105-6A-2V5 (SBY11894) cells. Pgk1 is shown as a loading control. Note that the cells for immunoprecipitation experiments were treated with benomyl, and protein levels for additional relevant panels are shown in Supplemental Figure S2.
Figure 4.
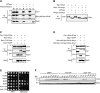
Identification of Bub1 phosphorylation sites required for the spindle checkpoint and Mad1 association. (A) The Mad1–kinetochore interaction requires Bub1 phosphorylation. λPPase treatment releases Mad1 from purified Bub1(M)-Spc105-6A kinetochore particles. Kinetochore particles (SBY11209) were immobilized on α-Flag beads and treated with λPPase with or without phosphatase inhibitors or without phosphatase. (IP) Starting input; (B) bound; (U) unbound. (*) An apparent degradation product of Bub1(M)-Spc105-6A corresponding to Spc105-6A migration. The immunoblots were probed with α-Flag, α-myc, or α-GFP antibodies. (B) The Bub1-15A mutant protein shows reduced phosphorylation in vivo. Bub1-9myc (SBY11764) or Bub1-15A-9myc (SBY11766) was immunoprecipitated from benomyl-treated cells and eluted directly or after treatment with λPPase with or without phosphatase inhibitors. The immunoblot was probed with α-myc. (C) Mad1 does not associate with kinetochore particles containing the Bub1-15A mutant protein. Kinetochore particles were immunoprecipitated from benomyl-treated cells with α-Flag beads. The strains contained untagged Mad1 (SBY8253), Mad1-3GFP (SBY8415), Mad1-3GFP Bub1-9myc (SBY11764), or Mad1-3GFP Bub1-15A-9myc (SBY11766). Immunoblots were probed with α-Flag, α-myc, or α-GFP antibodies. (D) Mad1 association with Bub1(M)-Spc105-6A kinetochores requires Bub1 phosphorylation sites. Dsn1-6His-3Flag was purified from a strain with untagged Mad1 (SBY10267) or strains with Mad1-3GFP and Bub1(M)-Spc105-6A-9myc (SBY11209) or Bub1(M)-15A-Spc105-6A-9myc (SBY11415). Immunoblots were probed with α-Flag, α-myc, or α-GFP antibodies. (E) bub1-15A mutant cells are benomyl-sensitive. Fivefold serial dilutions of the following strains were grown on YPD or YPD + 7.5 μg/mL benomyl: wild type (WT) (SBY8253), mad2Δ (SBY292), bub1Δ (SBY10596), and two independent transformants of the bub1Δ strain (SBY10596) with either BUB1-9myc (pSB1983) or bub1-15A-9myc (pSB1984). (F) bub1-15A mutant cells are defective in the spindle checkpoint. BUB1-6His (SBY11340), bub1ΔM-6His (SBY11342), and bub1-15A-6His (SBY11341) strains were released from a G1 arrest into medium containing nocodazole. Pds1-18myc levels were analyzed by α-myc immunoblotting at the indicted time points (in minutes). Pgk1 is shown as a loading control.
Figure 5.
Mps1 phosphorylation of Bub1 recruits Mad1 to the kinetochore. (A) Bub1 kinase activity is not required for Mad1 kinetochore association. Kinetochore particles were immunoprecipitated from benomyl-treated cultures from strains with untagged Mad1 (SBY8253), Mad1-3GFP (SBY8415), or Mad1-3GFP Bub1Δkinase (SBY11006). Note that a Mad1-3GFP degradation product is observed in this strain (*). Immunoblots were probed with α-Flag or α-GFP antibodies. (B) Mps1 phosphorylates the middle region of Bub1 in vitro. Kinase assays were performed with purified MBP or MBP fusions plus/minus recombinant GST-Mps1 bound to glutathione resin. Phosphorylation was detected by autoradiography (top), and total protein was detected by Coomassie staining (bottom). The 15A fragment phosphorylation signal was reduced 26% relative to wild type in this experiment. (C) Mps1 overexpression leads to Mad1–Bub1 association in G1-arrested cells. Bub1-3Flag was immunoprecipitated from cells arrested in G1 and treated with glucose or galactose. (Left to right) SBY11541 (pGAL-MPS1 MAD1-13myc), SBY11490 (pGAL-MPS1 BUB1-3Flag), SBY10532 (BUB1-3Flag MAD1-13myc), and SBY11488 (pGAL-MPS1 BUB1-3Flag MAD1-13myc). Immunoblots were probed with α-Flag or α-myc antibodies. (D) Schematic of experiment to determine whether Mps1 phosphorylation recruits Mad1 to kinetochores in vitro. Bub1(M)-Spc105-6A-9myc kinetochores [KT-Bub1(M)] bound to beads were treated with phosphatase and then incubated with ATP to allow Mps1 phosphorylation. The beads were then added to yeast extract containing Mad1-3GFP and washed, and the kinetochores were eluted. (E) Mps1 phosphorylation of kinetochore particles recruits Mad1 and depends on Bub1 phosphorylation sites. Bead-bound Bub1(M)-Spc105-6A-9myc (SBY11149) or Bub1(M)-15A-Spc105-6A-9myc kinetochores (SBY11325) were used for the experiment outlined in D. Immunoblots were probed with α-Flag or α-GFP antibodies. (F) Mad2, but not Bub1 or Bub3, is needed to enable Mad1 recruitment to Bub1(M)-Spc105-6A-9myc kinetochore particles phosphorylated by Mps1. Kinetochore particles were purified from wild-type (WT) (SBY8253) or bub1(M)-spc105-6A-9myc (SBY11149) strains and then treated as in D to analyze Mad1-3GFP binding from either wild-type (SBY8416), _bub1_Δ (SBY11464), _bub3_Δ (SBY11418), or _mad2_Δ (SBY11389) extracts. Immunoblots were probed with α-Flag or α-GFP antibodies. Binding of Mad1-3GFP to wild-type kinetochores in the +ATP condition is likely due to recruitment of preassociated Bub1–Mad1 complex through Spc105 phosphorylation. (G) Mad1 recruitment to kinetochore particles depends on Mps1 activity. Bub1(M)-Spc105-6A-9myc kinetochores were purified from either wild-type (SBY11149) or mps1-as1 (SBY11486) strains and used for the experiment outlined in D. mps1-as1 kinetochores were treated with the specific inhibitor 1NM-PP1. Immunoblots were probed with α-Flag or α-GFP antibodies.
Figure 6.
Recombinantly expressed Bub1 and Mad1 interact and require Mps1 and Mad2. (A) Recombinantly expressed Bub1 and Mad1 interact. The indicated proteins were coexpressed in bacteria and purified using S-protein resin. Note that there are multiple Mad1 and Bub1 species, presumably due to degradation, and the phosphorylation of the Bub1 fragments by Mps1 leads to a large mobility shift that is difficult to resolve by SDS-PAGE. The immunoblots were probed with α-S-tag, α-Flag, or α-V5 antibodies. (B) Bub1 phosphorylation, but not Mad1 phosphorylation, is required for in vitro binding of Bub1 and Mad1. Mad1(C)-3Flag was purified by itself or together with 2V5-Mad2 in the presence or absence of Mps1 expression. (*) Mps1 coexpression. The indicated Bub1(M)-S-tag fragments were immobilized on S-protein beads and incubated with the Mad1 proteins in vitro to assay for binding. The immunoblots were probed with α-S-tag or α-Flag antibodies.
Figure 7.
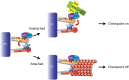
Model. Mps1 activity during mitosis phosphorylates Spc105 to recruit the Bub1/3 complex (London et al. 2012; Shepperd et al. 2012; Yamagishi et al. 2012). Mps1 then phosphorylates Bub1 at unattached kinetochores to recruit Mad1/2 and activate the downstream checkpoint response. Microtubule attachment opposes Mps1 activity, possibly by promoting Mps1 dissociation from kinetochores, and results in Mad1 dissociation from kinetochores to silence the checkpoint (Jelluma et al. 2010).
Similar articles
- Synthetic Physical Interactions Map Kinetochore-Checkpoint Activation Regions.
Ólafsson G, Thorpe PH. Ólafsson G, et al. G3 (Bethesda). 2016 Aug 9;6(8):2531-42. doi: 10.1534/g3.116.031930. G3 (Bethesda). 2016. PMID: 27280788 Free PMC article. - Dual mechanisms regulate the recruitment of spindle assembly checkpoint proteins to the budding yeast kinetochore.
Aravamudhan P, Chen R, Roy B, Sim J, Joglekar AP. Aravamudhan P, et al. Mol Biol Cell. 2016 Nov 7;27(22):3405-3417. doi: 10.1091/mbc.E16-01-0007. Epub 2016 May 11. Mol Biol Cell. 2016. PMID: 27170178 Free PMC article. - The Bub1-TPR Domain Interacts Directly with Mad3 to Generate Robust Spindle Checkpoint Arrest.
Leontiou I, London N, May KM, Ma Y, Grzesiak L, Medina-Pritchard B, Amin P, Jeyaprakash AA, Biggins S, Hardwick KG. Leontiou I, et al. Curr Biol. 2019 Jul 22;29(14):2407-2414.e7. doi: 10.1016/j.cub.2019.06.011. Epub 2019 Jun 27. Curr Biol. 2019. PMID: 31257143 Free PMC article. - Orchestration of the spindle assembly checkpoint by CDK1-cyclin B1.
Hayward D, Alfonso-Pérez T, Gruneberg U. Hayward D, et al. FEBS Lett. 2019 Oct;593(20):2889-2907. doi: 10.1002/1873-3468.13591. Epub 2019 Sep 13. FEBS Lett. 2019. PMID: 31469407 Review.
Cited by
- Synthetic physical interactions map kinetochore regulators and regions sensitive to constitutive Cdc14 localization.
Ólafsson G, Thorpe PH. Ólafsson G, et al. Proc Natl Acad Sci U S A. 2015 Aug 18;112(33):10413-8. doi: 10.1073/pnas.1506101112. Epub 2015 Aug 3. Proc Natl Acad Sci U S A. 2015. PMID: 26240346 Free PMC article. - Mad1 promotes chromosome congression by anchoring a kinesin motor to the kinetochore.
Akera T, Goto Y, Sato M, Yamamoto M, Watanabe Y. Akera T, et al. Nat Cell Biol. 2015 Sep;17(9):1124-33. doi: 10.1038/ncb3219. Epub 2015 Aug 10. Nat Cell Biol. 2015. PMID: 26258632 - Mph1/MPS1 checks in at the kinetochore.
Hervas-Aguilar A, Millar JB. Hervas-Aguilar A, et al. Cell Cycle. 2016 May 18;15(10):1313-4. doi: 10.1080/15384101.2016.1159888. Epub 2016 Apr 22. Cell Cycle. 2016. PMID: 27105354 Free PMC article. No abstract available. - Mps1 regulates spindle morphology through MCRS1 to promote chromosome alignment.
Yang H, Zhang F, Huang CJ, Liao J, Han Y, Hao P, Chu Y, Lu X, Li W, Yu H, Kang J. Yang H, et al. Mol Biol Cell. 2019 Apr 15;30(9):1060-1068. doi: 10.1091/mbc.E18-09-0546. Epub 2019 Feb 20. Mol Biol Cell. 2019. PMID: 30785839 Free PMC article. - Kinetochore-bound Mps1 regulates kinetochore-microtubule attachments via Ndc80 phosphorylation.
Sarangapani KK, Koch LB, Nelson CR, Asbury CL, Biggins S. Sarangapani KK, et al. J Cell Biol. 2021 Dec 6;220(12):e202106130. doi: 10.1083/jcb.202106130. Epub 2021 Oct 14. J Cell Biol. 2021. PMID: 34647959 Free PMC article.
References
- Abrieu A, Magnaghi-Jaulin L, Kahana JA, Peter M, Castro A, Vigneron S, Lorca T, Cleveland DW, Labbe JC 2001. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell 106: 83–93 - PubMed
- Bolanos-Garcia VM, Kiyomitsu T, D'Arcy S, Chirgadze DY, Grossmann JG, Matak-Vinkovic D, Venkitaraman AR, Yanagida M, Robinson CV, Blundell TL 2009. The crystal structure of the N-terminal region of BUB1 provides insight into the mechanism of BUB1 recruitment to kinetochores. Structure 17: 105–116 - PMC - PubMed
- Brady DM, Hardwick KG 2000. Complex formation between Mad1p, Bub1p and Bub3p is crucial for spindle checkpoint function. Curr Biol 10: 675–678 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases