Lung development: orchestrating the generation and regeneration of a complex organ - PubMed (original) (raw)
Review
Lung development: orchestrating the generation and regeneration of a complex organ
Michael Herriges et al. Development. 2014 Feb.
Abstract
The respiratory system, which consists of the lungs, trachea and associated vasculature, is essential for terrestrial life. In recent years, extensive progress has been made in defining the temporal progression of lung development, and this has led to exciting discoveries, including the derivation of lung epithelium from pluripotent stem cells and the discovery of developmental pathways that are targets for new therapeutics. These discoveries have also provided new insights into the regenerative capacity of the respiratory system. This Review highlights recent advances in our understanding of lung development and regeneration, which will hopefully lead to better insights into both congenital and acquired lung diseases.
Keywords: Branching morphogenesis; Epigenetics; Lung; Regeneration.
Figures
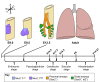
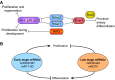
Fig. 1.
Overview of the stages of lung development. Lung endoderm specification begins at ∼E9.0 on the ventral side of the anterior foregut endoderm (yellow) where initiation of Nkx2.1 expression commences. By E9.5-E10.0, the formation of the trachea is observed and the embryonic stage of lung development begins and ends at E12.5. The other stages of lung development and the time period in which they occur are displayed. A, anterior; D, dorsal; L, left; P, posterior; R, right; V, ventral.
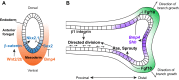
Fig. 2.
Specification and early development of the lung endoderm. (A) The lung endoderm (marked by Nkx2.1 expression, blue) is first specified on the ventral side of the anterior foregut at E9.0. Wnt2/2b and Bmp4 signaling (indicated in orange???) from the surrounding mesoderm is required for this specification and for patterning of the anterior foregut in a ventral-dorsal manner. Wnt2 and Wnt2b signal via β-catenin to promote the expression of Nkx2.1 whereas Bmp4 represses the inhibitory action of Sox2 on Nkx2.1 expression. (B) During lung development, Fgf10 is essential for branching morphogenesis and is expressed in the mesenchyme surrounding developing branch points (Fgf10 expression indicated in green). This expression is restricted by epithelial expression of genes such Shh and Bmp4 (purple). Fgf10 also directs the orientation of epithelial cell division through regulation of Ras/Sprouty, setting up the appropriate direction of branch growth.
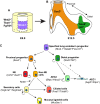
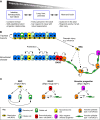
Fig. 3.
Progenitors and cell lineages within the developing lung. (A) Early Nkx2.1+ lung endoderm progenitors (purple) are surrounded by lung mesenchyme (yellow), which is marked by the expression of Wnt2, Tbx4 and Fgf10. (B,C) This Nkx2.1-positive endoderm gives rise to both Sox2+ proximal progenitors and Sox9+/Id2+ distal progenitors, which in turn give rise to distinct sets of differentiated cells. The proximal progenitors give rise to neuroendocrine (NE) cells, secretory cells, ciliated cells and mucosal cells that all populate the conducting airways. By contrast, the distal progenitors give rise to type I and type 2 alveolar epithelial cells (AEC1 and AEC2) that populate the alveoli. Markers expressed in each cell type are indicated in parentheses.
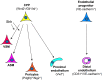
Fig. 4.
The origin of the pulmonary vasculature and other lung mesodermal derivatives. The majority of lung mesodermal lineages, including vascular smooth muscle (VSM), airway smooth muscle (ASM), pericytes and proximal endothelium are derived from Wnt2+ cardiopulmonary progenitors (CPPs). Hedgehog signaling regulates the differentiation of CPPs into smooth muscle. By contrast, distal vascular endothelial cells that generate the majority of the alveolar vascular plexus are derived from VE-cadherin+ progenitors. Thus, the pulmonary vasculature is generated using a multi-lineage approach. Markers expressed in each cell type are indicated in parentheses.
Fig. 5.
Epigenetic factors regulate lung development and regeneration. (A) Hdac1/2 activity promotes proliferation during development and regeneration through inhibition of tumor suppressors, such as Rb1, p16 and p21. During development, Hdac1/2 also promote proximal airway differentiation through inhibition of Bmp4. (B) miRNAs that are expressed early in lung development (miR302/367 and miR17∼92) promote proliferation and inhibit differentiation of lung epithelium, whereas those expressed at later stages (miR34/449 and miR375) inhibit proliferation and promote differentiation.
Fig. 6.
Regional progenitors contribute to lung repair and regeneration. (A) Different organs have different capacities for self-renewal and regeneration. The lung, although largely quiescent in the adult, is capable of regenerating in response to injury. (B) Basal stem cells (BSCs) can regenerate both secretory and ciliated epithelium of the trachea and proximal bronchi. Some evidence indicates that BSCs can also generate alveolar epithelium after extreme injury. In the bronchiolar region, secretory cells can regenerate themselves and ciliated cells after injury. Neuroendocrine cells can also regenerate secretory and ciliated cells after loss through injury, although this capacity maybe limited. Bronchioalveolar stem cells (BASCs) lie at the border between the bronchiolar and alveolar regions and are marked by co-expression of Sftpc and Scgb1a1. BASCs are thought to contribute to both bronchiolar and alveolar regions after injury. Within the alveolar region, Sftpc+ AEC2 cells have been shown to self-renew and differentiate into AEC1 cells after injury and during homeostasis. (C) Relationship between the various stem/progenitor lineages and their differentiated progeny. Relationships that are predicted to possibly occur but have not yet been demonstrated are shown with dashed arrows.
Similar articles
- Generation of functional lungs via conditional blastocyst complementation using pluripotent stem cells.
Mori M, Furuhashi K, Danielsson JA, Hirata Y, Kakiuchi M, Lin CS, Ohta M, Riccio P, Takahashi Y, Xu X, Emala CW, Lu C, Nakauchi H, Cardoso WV. Mori M, et al. Nat Med. 2019 Nov;25(11):1691-1698. doi: 10.1038/s41591-019-0635-8. Epub 2019 Nov 7. Nat Med. 2019. PMID: 31700187 Free PMC article. - Organ regeneration based on developmental biology: past and future.
Takeo M, Tsuji T. Takeo M, et al. Curr Opin Genet Dev. 2018 Oct;52:42-47. doi: 10.1016/j.gde.2018.05.008. Epub 2018 Jun 5. Curr Opin Genet Dev. 2018. PMID: 29883895 Review. - Use of three-dimensional organoids and lung-on-a-chip methods to study lung development, regeneration and disease.
Gkatzis K, Taghizadeh S, Huh D, Stainier DYR, Bellusci S. Gkatzis K, et al. Eur Respir J. 2018 Nov 29;52(5):1800876. doi: 10.1183/13993003.00876-2018. Print 2018 Nov. Eur Respir J. 2018. PMID: 30262579 Review. - Signaling networks regulating development of the lower respiratory tract.
Ornitz DM, Yin Y. Ornitz DM, et al. Cold Spring Harb Perspect Biol. 2012 May 1;4(5):a008318. doi: 10.1101/cshperspect.a008318. Cold Spring Harb Perspect Biol. 2012. PMID: 22550231 Free PMC article. Review. - Stem cells and lung regeneration.
Parekh KR, Nawroth J, Pai A, Busch SM, Senger CN, Ryan AL. Parekh KR, et al. Am J Physiol Cell Physiol. 2020 Oct 1;319(4):C675-C693. doi: 10.1152/ajpcell.00036.2020. Epub 2020 Aug 12. Am J Physiol Cell Physiol. 2020. PMID: 32783658 Free PMC article. Review.
Cited by
- hPSC-derived lung and intestinal organoids as models of human fetal tissue.
Aurora M, Spence JR. Aurora M, et al. Dev Biol. 2016 Dec 15;420(2):230-238. doi: 10.1016/j.ydbio.2016.06.006. Epub 2016 Jun 7. Dev Biol. 2016. PMID: 27287882 Free PMC article. Review. - Association between clinical respiratory signs, lung lesions detected by thoracic ultrasonography and growth performance in pre-weaned dairy calves.
Cuevas-Gómez I, McGee M, Sánchez JM, O'Riordan E, Byrne N, McDaneld T, Earley B. Cuevas-Gómez I, et al. Ir Vet J. 2021 Mar 25;74(1):7. doi: 10.1186/s13620-021-00187-1. Ir Vet J. 2021. PMID: 33766106 Free PMC article. - Growth performance and hematological changes of weaned beef calves diagnosed with respiratory disease using respiratory scoring and thoracic ultrasonography.
Cuevas-Gómez I, McGee M, McCabe M, Cormican P, O'Riordan E, McDaneld T, Earley B. Cuevas-Gómez I, et al. J Anim Sci. 2020 Nov 1;98(11):skaa345. doi: 10.1093/jas/skaa345. J Anim Sci. 2020. PMID: 33095858 Free PMC article. - Human airway organoid engineering as a step toward lung regeneration and disease modeling.
Tan Q, Choi KM, Sicard D, Tschumperlin DJ. Tan Q, et al. Biomaterials. 2017 Jan;113:118-132. doi: 10.1016/j.biomaterials.2016.10.046. Epub 2016 Oct 28. Biomaterials. 2017. PMID: 27815996 Free PMC article. - TBX2-positive cells represent a multi-potent mesenchymal progenitor pool in the developing lung.
Wojahn I, Lüdtke TH, Christoffels VM, Trowe MO, Kispert A. Wojahn I, et al. Respir Res. 2019 Dec 23;20(1):292. doi: 10.1186/s12931-019-1264-y. Respir Res. 2019. PMID: 31870435 Free PMC article.
References
- Anderson-Berry A., O’Brien E. A., Bleyl S. B., Lawson A., Gundersen N., Ryssman D., Sweeley J., Dahl M. J., Drake C. J., Schoenwolf G. C., et al. (2005). Vasculogenesis drives pulmonary vascular growth in the developing chick embryo. Dev. Dyn. 233, 145–153 - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL071589/HL/NHLBI NIH HHS/United States
- R01 HL087825/HL/NHLBI NIH HHS/United States
- HL110942/HL/NHLBI NIH HHS/United States
- U01 HL110942/HL/NHLBI NIH HHS/United States
- HL071589/HL/NHLBI NIH HHS/United States
- HL087825/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources