Stable MCC binding to the APC/C is required for a functional spindle assembly checkpoint - PubMed (original) (raw)
Stable MCC binding to the APC/C is required for a functional spindle assembly checkpoint
Jamin B Hein et al. EMBO Rep. 2014 Mar.
Abstract
The spindle assembly checkpoint (SAC) delays progression into anaphase until all chromosomes have aligned on the metaphase plate by inhibiting Cdc20, the mitotic co-activator of the APC/C. Mad2 and BubR1 bind and inhibit Cdc20, thereby forming the mitotic checkpoint complex (MCC), which can bind stably to the APC/C. Whether MCC formation per se is sufficient for a functional SAC or MCC association with the APC/C is required remains unclear. Here, we analyze the role of two conserved motifs in Cdc20, IR and C-Box, in binding of the MCC to the APC/C. Mutants in both motifs assemble the MCC normally, but IR motif integrity is particularly important for stable binding to the APC/C. Cells expressing Cdc20 with a mutated IR motif have a compromised SAC, as uninhibited Cdc20 can compete with the MCC for APC/C binding and activate it. We thus show that stable MCC association with the APC/C is critical for a functional SAC.
Figures
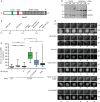
Figure 1
The IR motif and C-Box of Cdc20 are required for mitotic progression.
- Schematic representation of Cdc20 with important functional motifs investigated in this study. Indicated are the seven WD40 repeats (grey), the C-Box (green) and MIM (Mad2 interacting motif) (red) in the unstructured N-terminal part and the IR motif (blue) in the C-terminus.
- Blot of Cdc20 RNAi-treated cells and control-treated cells is shown with the indicated amount of total extract loaded.
- Experimental setup of RNAi rescue time-lapse experiments.
- Representative DIC and fluorescence images of RNAi rescue time-lapse experiments. Ctrl RNAi-treated cells progress through mitosis normally with a median time of 54 min. Cdc20 RNAi-treated cells arrest at metaphase with a median time of 84 min, which is a significant increase compared to control (P < 0.0001). This phenotype is only rescued in Cdc20 WT-expressing cells, not in Cdc20 ΔC-Box-, Cdc20 ΔC-Box/R499E-or Cdc20 R499E-expressing cells.
- Quantification of mitotic timing (NEBD to anaphase, arrest or apoptosis). N > 47 per conditions shown as a box-and-whisker plot with the median indicated by a line in the box representing the lower and upper quartile. Mann-Whitney test was performed for statistical analysis (_P_-value: ****P < 0.0001, n.s., non significant). Only cells recorded for at least 2 h were included in the analysis.
Source data are available online for this figure.
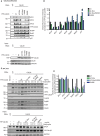
Figure 2
MCC complex formation is not affected by mutation of the C-box or IR motif.
- Endogenous Cdc20 was depleted by RNAi, cells were synchronized for 24 h in thymidine, released into nocodazole for 11 h and harvested by mitotic shake-off. 24 h prior to shake-off, expression of the indicated constructs was induced by doxycycline (10 ng/ml) and YFP-Cdc20 purified. The binding of MCC and APC/C components was analyzed by Western blot.
- Quantification of MCC and APC/C components normalized to YFP-Cdc20 signal and with Cdc20 WT set to 1. Mean of two independent experiments shown with the black squares showing the values obtained in the two experiments.
- Purification of the APC/C complex using an APC4 monoclonal antibody. The cells were synchronized as in (A) and either induced with 0.5 ng/ml or 10 ng/ml doxycycline for 24 h. The binding of MCC components and Cdc20 proteins was analyzed by Western blot.
- Quantification of three independent experiments as in (C) with the mean and standard deviation shown.
Source data are available online for this figure.
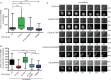
Figure 3
The IR motif is required for a functional SAC.
- Cells expressing the indicated Cdc20 proteins were monitored by time-lapse microscopy in the presence of endogenous Cdc20 and the time from NEBD to anaphase measured. Cells expressing the indicated Cdc20 proteins were synchronized by a double thymidine block. 24 h prior to imaging doxycycline (10 ng/ml) was added to induce protein expression and cells were filmed every 6 min by time-lapse microscopy for 18 h. At least 146 cells were analyzed per condition shown as a box-and-whisker plot with the median indicated by a line in the box representing the lower and upper quartile. Mann–Whitney test was performed for statistical analysis (_P_-value: ****P < 0.0001, ns, non significant).
- Similar experiment as in (A) but in the presence of 30 ng/ml nocodazole. Time from anaphase to mitotic exit, arrest or apoptosis is measured. N > 35 shown as a box-and-whisker plot with the median indicated by a line in the box representing the lower and upper quartile. Mann–Whitney test was performed for statistical analysis (_P_-value: ****P < 0.0001, ns, non significant).
- Representative DIC and fluorescent images for the experiments quantified in (B).
Source data are available online for this figure.
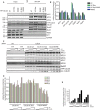
Figure 4
Endogenous Cdc20 can bind APC/C in Cdc20 R499E cells.
- The APC/C complex was purified from nocodazole-arrested cells using an APC4 monoclonal antibody and expressing the indicated exogenous Cdc20 proteins. The binding of endogenous and exogenous Cdc20 as well as MCC components was analyzed by Western blotting.
- Quantification of the experiment described in (A) with the signal normalized to APC4 and to Cdc20 WT. The mean and standard deviation from 3 independent experiments is shown.
- From the indicated YFP-Cdc20-expressing cells a mitotic lysate was prepared and increasing concentrations of recombinant Cdc20 added corresponding to 5-, 25-and 50-fold above endogenous Cdc20 levels respectively. Following incubation for 1 h at 37°C the YFP-tagged Cdc20 was purified and the amount of recombinant Cdc20 as well as APC/C subunits bound analyzed by Western blot.
- Quantification of the indicated APC subunits normalized to YFP-Cdc20 with increasing concentrations of recombinant Cdc20 added.
- Quantification of recombinant Cdc20 bound relative to YFP-Cdc20.
Source data are available online for this figure.
Similar articles
- The Mitotic Checkpoint Complex Requires an Evolutionary Conserved Cassette to Bind and Inhibit Active APC/C.
Di Fiore B, Wurzenberger C, Davey NE, Pines J. Di Fiore B, et al. Mol Cell. 2016 Dec 15;64(6):1144-1153. doi: 10.1016/j.molcel.2016.11.006. Epub 2016 Dec 8. Mol Cell. 2016. PMID: 27939943 Free PMC article. - Role of ubiquitylation of components of mitotic checkpoint complex in their dissociation from anaphase-promoting complex/cyclosome.
Sitry-Shevah D, Kaisari S, Teichner A, Miniowitz-Shemtov S, Hershko A. Sitry-Shevah D, et al. Proc Natl Acad Sci U S A. 2018 Feb 20;115(8):1777-1782. doi: 10.1073/pnas.1720312115. Epub 2018 Feb 5. Proc Natl Acad Sci U S A. 2018. PMID: 29432156 Free PMC article. - The mitotic checkpoint complex binds a second CDC20 to inhibit active APC/C.
Izawa D, Pines J. Izawa D, et al. Nature. 2015 Jan 29;517(7536):631-4. doi: 10.1038/nature13911. Epub 2014 Nov 12. Nature. 2015. PMID: 25383541 Free PMC article. - The spindle checkpoint: how do cells delay anaphase onset?
Sczaniecka MM, Hardwick KG. Sczaniecka MM, et al. SEB Exp Biol Ser. 2008;59:243-56. SEB Exp Biol Ser. 2008. PMID: 18368927 Review. - Dual inhibition of Cdc20 by the spindle checkpoint.
Chen RH. Chen RH. J Biomed Sci. 2007 Jul;14(4):475-9. doi: 10.1007/s11373-007-9157-3. Epub 2007 Mar 17. J Biomed Sci. 2007. PMID: 17370142 Review.
Cited by
- Heat stress during male meiosis impairs cytoskeletal organization, spindle assembly and tapetum degeneration in wheat.
Fábián A, Péntek BK, Soós V, Sági L. Fábián A, et al. Front Plant Sci. 2024 Jan 8;14:1314021. doi: 10.3389/fpls.2023.1314021. eCollection 2023. Front Plant Sci. 2024. PMID: 38259921 Free PMC article. - Hematopoietic PBX-interacting protein is a substrate and an inhibitor of the APC/C-Cdc20 complex and regulates mitosis by stabilizing cyclin B1.
Khumukcham SS, Samanthapudi VSK, Penugurti V, Kumari A, Kesavan PS, Velatooru LR, Kotla SR, Mazumder A, Manavathi B. Khumukcham SS, et al. J Biol Chem. 2019 Jun 28;294(26):10236-10252. doi: 10.1074/jbc.RA118.006733. Epub 2019 May 17. J Biol Chem. 2019. PMID: 31101654 Free PMC article. - Transcriptional and post-transcriptional regulation of Cdc20 during the spindle assembly checkpoint in S. cerevisiae.
Wang R, Burton JL, Solomon MJ. Wang R, et al. Cell Signal. 2017 May;33:41-48. doi: 10.1016/j.cellsig.2017.02.003. Epub 2017 Feb 9. Cell Signal. 2017. PMID: 28189585 Free PMC article. - CCDC68 Maintains Mitotic Checkpoint Activation by Promoting CDC20 Integration into the MCC.
Li Q, Chen Q, Zheng T, Wang F, Teng J, Zhou H, Chen J. Li Q, et al. Adv Sci (Weinh). 2024 Sep;11(35):e2406009. doi: 10.1002/advs.202406009. Epub 2024 Jul 17. Adv Sci (Weinh). 2024. PMID: 39018254 Free PMC article. - Regulation of mitotic progression by the spindle assembly checkpoint.
Lischetti T, Nilsson J. Lischetti T, et al. Mol Cell Oncol. 2015 Jan 30;2(1):e970484. doi: 10.4161/23723548.2014.970484. eCollection 2015 Jan-Mar. Mol Cell Oncol. 2015. PMID: 27308407 Free PMC article. Review.
References
- Lara-Gonzalez P, Westhorpe FG, Taylor SS. The spindle assembly checkpoint. Curr Biol. 2012;22:R966–R980. - PubMed
- Pines J. Cubism and the cell cycle: the many faces of the APC/C. Nat Rev Mol Cell Biol. 2011;12:427–438. - PubMed
- Vodermaier HC, Gieffers C, Maurer-Stroh S, Eisenhaber F, Peters J-M. TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1. Curr Biol. 2003;13:1459–1468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources