PGE2-driven expression of c-Myc and oncomiR-17-92 contributes to apoptosis resistance in NSCLC - PubMed (original) (raw)
doi: 10.1158/1541-7786.MCR-13-0377. Epub 2014 Jan 27.
Rebecca Kusko, Tristan Grogan, James O'Hearn, Karen L Reckamp, Tonya C Walser, Edward B Garon, Marc E Lenburg, Sherven Sharma, Avrum E Spira, David Elashoff, Steven M Dubinett
Affiliations
- PMID: 24469837
- PMCID: PMC4020971
- DOI: 10.1158/1541-7786.MCR-13-0377
PGE2-driven expression of c-Myc and oncomiR-17-92 contributes to apoptosis resistance in NSCLC
Kostyantyn Krysan et al. Mol Cancer Res. 2014 May.
Abstract
Aberrant expression of microRNAs (miRNA) with oncogenic capacities (oncomiRs) has been described for several different malignancies. The first identified oncomiR, miR-17-92, is frequently overexpressed in a variety of cancers and its targets include the tumor suppressor PTEN. The transcription factor c-Myc (MYC) plays a central role in proliferative control and is rapidly upregulated upon mitogenic stimulation. Expression of c-Myc is frequently deregulated in tumors, facilitating proliferation and inhibiting terminal differentiation. The c-Myc-regulated network comprises a large number of transcripts, including those encoding miRNAs. Here, prostaglandin E2 (PGE2) exposure rapidly upregulates the expression of the MYC gene followed by the elevation of miR-17-92 levels, which in turn suppresses PTEN expression, thus enhancing apoptosis resistance in non-small cell lung cancer (NSCLC) cells. Knockdown of MYC expression or the miR-17-92 cluster effectively reverses this outcome. Similarly, miR-17-92 levels are significantly elevated in NSCLC cells ectopically expressing COX-2. Importantly, circulating miR-17-92 was elevated in the blood of patients with lung cancer as compared with subjects at risk for developing lung cancer. Furthermore, in patients treated with celecoxib, miR-17-92 levels were significantly reduced. These data demonstrate that PGE2, abundantly produced by NSCLC and inflammatory cells in the tumor microenvironment, is able to stimulate cell proliferation and promote resistance to pharmacologically induced apoptosis in a c-Myc and miR-17-92-dependent manner.
Implications: This study describes a novel mechanism, involving c-Myc and miR-17-92, which integrates cell proliferation and apoptosis resistance.
©2014 AACR.
Figures
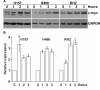
Figure 1. Induction of c-myc expression in NSCLC cells by PGE2
H157, H460 and RH2 NSCLC cells were serum deprived for 3 hours and treated with 5 μg/ml PGE2 in the fresh serum-free medium for 0, 1, 2 or 3 hours. The cells were then harvested and total RNA and proteins were isolated. Upregulation of c-myc following the PGE2 treatment was detected at transcriptional and protein levels starting at 60 minutes post treatment and for the entire 3 hours of treatment. (A) Proteins were separated by SDS-PAGE and c-myc was detected by immunoblotting. Representative of two independent experiments is shown. (B) C-myc mRNA expression was assessed by TaqMan qRT-PCR. GUS (H157 and H460) or B2M (RH2) expression was used as internal control. Expression was normalized to that observed in the untreated group (0 h).
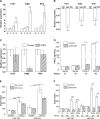
Figure 2. Expression of miR-19b is upregulated by PGE2
(A) MiR-19b is upregulated by PGE2 treatment in a time-dependent manner in NSCLC cells. H157, H460 and RH2 cells were serum deprived for 3 hours and treated with 5 μg/ml PGE2 in the fresh serum-free medium for 1, 2 or 3 hours followed by total RNA isolation and TaqMan qRT-PCR for miR-19b and RNU44 (internal control). (B) Basal miR-19b levels are reduced in c-myc knockdown (KD) H157, H460 and RH2 cells compared to non-silencing construct-transduced control cells (NC). (C) C-myc knockdown abolishes PGE2-driven upregulation of miR-19b. C-myc knockdown cells were serum deprived and treated with the diluent control or 5 μg/ml PGE2 in the fresh serum-free medium for 3 hours. (D) PGE2 levels are elevated in H157 cells with ectopic COX-2 expression compared to vector control cells and are significantly reduced by incubation with 2.5 μM celecoxib for 24 or 48 hours. PGE2 data was normalized to the total RNA concentration. (E) Ectopic COX-2 expression leads to the upregulation of the miR-17-92 cluster. Q-RT-PCR was performed on RNA from vector control and COX-2 overexpressing H157 cells. (F) Celecoxib treatment (2.5 μM for 24 or 48 hours) leads to the miR-17-92 downregulation in COX-2 overexpressing cells (COX) compared to vector control (V) as determined by q-RT-PCR.
Figure 3. Mir-17-92 cluster is upregulated in circulating miRNA from serum of lung cancer patients
(A) Mir-17-92 is highly elevated in cancer patients compared to subjects at high risk for developing lung cancer. Analysis of mir-17-92 expression in plasma of 30 high-risk controls and 30 advanced stage NSCLC patients by RNA-seq. Results are fold change in advanced stage lung cancer vs. high-risk controls. (B) Celecoxib treatment reduces the levels of the circulating plasma miR-19b and miR-92a in NSCLC patients. Results are mean relative expression ± SEM, p-values computed by a paired samples t-test.
Figure 4. PTEN is negatively regulated by PGE2
Total RNA was isolated from H157, H460 and RH2 cells and probed for PTEN expression using TaqMan system. Expression was normalized to that observed in the control or untreated groups. (A) PGE2 reduces PTEN mRNA levels. The parental H157, H460 and RH2 cells were serum deprived and treated with the diluent control or 5 μg/ml PGE2 in the fresh serum-free medium for 0, 1, 2 or 3 hours. (B) C-myc knockdown reverses PGE2-driven PTEN downregulation. Non-silencing shRNA construct-transduced control cells and c-myc knockdown cells were plated in 6-well plates and incubated overnight. The cells were then serum deprived and treated with the diluent control (C) or 5 μg/ml PGE2 (PGE2) in the fresh serum-free medium for 3 hours. PGE2 treatment significantly reduced PTEN expression in control cells but not in c-myc knockdown cells. (C) MiR-19b knockdown blocks c-myc-dependent downregulation of PTEN. MiR-19b expression was knocked down with anti-miR antisense RNA in H157, H460 and RH2 cells. The control cells were transfected with the Negative Control anti-miR. Twenty-four hours after the transfection, the cells were serum deprived and treated with the diluent or 5 μg/ml PGE2 for 0, 1, 2 or 3 hours. Negative control- but not anti-miR-19b-transfected cells demonstrated significant downregulation of PTEN by PGE2.
Figure 5. Mir-19b knockdown abolishes the anti-apoptotic effect of PGE2 in NSCLC cells
PGE2 protects the cells with intact miR-19b expression from apoptosis and this effect is reversed by miR-19 knockdown. The expression of miR-19b was knocked down with anti-miR antisense RNA in H157, H460 and RH2 cells (KD). Negative control anti-miR was used to transfect the control cells (NC). The cells were then pre-treated with 5 μg/ml PGE2 (PG) overnight followed by the induction of apoptosis by staurosporine (St) where applicable. The control cells were treated with the equivalent concentrations of the diluents (diluent control, DC). The cells were allowed to recover overnight (5 μg/ml PGE2 was added to the PGE2 groups) and then were harvested. The apoptosis levels were evaluated by flow cytometry utilizing Annexin V/PI staining. Percent difference (% diff.) indicates the difference in the total number of non-apoptotic cells per 10,000 gated cells (shown in the lower left quadrants) between cells treated with staurosporine alone or in combination with PGE2.
Similar articles
- MiR-376a suppresses the proliferation and invasion of non-small-cell lung cancer by targeting c-Myc.
Wang Y, Cong W, Wu G, Ju X, Li Z, Duan X, Wang X, Gao H. Wang Y, et al. Cell Biol Int. 2018 Jan;42(1):25-33. doi: 10.1002/cbin.10828. Epub 2017 Sep 11. Cell Biol Int. 2018. PMID: 28741879 - MicroRNA-184 Deregulated by the MicroRNA-21 Promotes Tumor Malignancy and Poor Outcomes in Non-small Cell Lung Cancer via Targeting CDC25A and c-Myc.
Lin TC, Lin PL, Cheng YW, Wu TC, Chou MC, Chen CY, Lee H. Lin TC, et al. Ann Surg Oncol. 2015 Dec;22 Suppl 3:S1532-9. doi: 10.1245/s10434-015-4595-z. Epub 2015 May 20. Ann Surg Oncol. 2015. PMID: 25990966 - MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC).
Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K, Yang GH. Zhang JG, et al. Clin Chim Acta. 2010 Jun 3;411(11-12):846-52. doi: 10.1016/j.cca.2010.02.074. Epub 2010 Mar 16. Clin Chim Acta. 2010. PMID: 20223231 - MYC: a multipurpose oncogene with prognostic and therapeutic implications in blood malignancies.
Ahmadi SE, Rahimi S, Zarandi B, Chegeni R, Safa M. Ahmadi SE, et al. J Hematol Oncol. 2021 Aug 9;14(1):121. doi: 10.1186/s13045-021-01111-4. J Hematol Oncol. 2021. PMID: 34372899 Free PMC article. Review. - [Research progress of the relationship between tumor suppressor gene PTEN and non-small cell lung cancer].
Li Y, Wang H. Li Y, et al. Zhongguo Fei Ai Za Zhi. 2014 Mar;17(3):260-4. doi: 10.3779/j.issn.1009-3419.2014.03.13. Zhongguo Fei Ai Za Zhi. 2014. PMID: 24667265 Free PMC article. Review. Chinese. No abstract available.
Cited by
- Small molecules targeting c-Myc oncogene: promising anti-cancer therapeutics.
Chen BJ, Wu YL, Tanaka Y, Zhang W. Chen BJ, et al. Int J Biol Sci. 2014 Sep 13;10(10):1084-96. doi: 10.7150/ijbs.10190. eCollection 2014. Int J Biol Sci. 2014. PMID: 25332683 Free PMC article. Review. - Monepantel antitumor activity is mediated through inhibition of major cell cycle and tumor growth signaling pathways.
Bahrami F, Mekkawy AH, Badar S, Morris DL, Pourgholami MH. Bahrami F, et al. Am J Cancer Res. 2021 Jun 15;11(6):3098-3110. eCollection 2021. Am J Cancer Res. 2021. PMID: 34249447 Free PMC article. - The Role of Interleukin 1β in the Pathogenesis of Lung Cancer.
Garon EB, Chih-Hsin Yang J, Dubinett SM. Garon EB, et al. JTO Clin Res Rep. 2020 Feb 11;1(1):100001. doi: 10.1016/j.jtocrr.2020.100001. eCollection 2020 Mar. JTO Clin Res Rep. 2020. PMID: 34589908 Free PMC article. Review. - The miR-17-92 cluster as a potential biomarker for the early diagnosis of gastric cancer: evidence and literature review.
Li H, Wu Q, Li T, Liu C, Xue L, Ding J, Shi Y, Fan D. Li H, et al. Oncotarget. 2017 Jul 11;8(28):45060-45071. doi: 10.18632/oncotarget.15023. Oncotarget. 2017. PMID: 28178677 Free PMC article. Review. - Co-Targeting PIM Kinase and PI3K/mTOR in NSCLC.
Moore G, Lightner C, Elbai S, Brady L, Nicholson S, Ryan R, O'Sullivan KE, O'Byrne KJ, Blanco-Aparicio C, Cuffe S, O'Neill M, Heavey S, Finn SP, Gately K. Moore G, et al. Cancers (Basel). 2021 Apr 29;13(9):2139. doi: 10.3390/cancers13092139. Cancers (Basel). 2021. PMID: 33946744 Free PMC article.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2010;144:646–74. - PubMed
- Huang M, Stolina M, Sharma S, Mao JT, Zhu L, Miller PW, et al. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 1998;58:1208–16. - PubMed
- Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, et al. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–45. - PubMed
- Krysan K, Dalwadi H, Sharma S, Pold M, Dubinett S. Cyclooxygenase 2-dependent expression of survivin is critical for apoptosis resistance in non-small cell lung cancer. Cancer Res. 2004;64:6359–62. - PubMed
- Krysan K, Reckamp KL, Dalwadi H, Sharma S, Rozengurt E, Dohadwala M, et al. Prostaglandin E2 activates mitogen-activated protein kinase/Erk pathway signaling and cell proliferation in non-small cell lung cancer cells in an epidermal growth factor receptor-independent manner. Cancer Res. 2005;65:6275–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1TR000124/TR/NCATS NIH HHS/United States
- CA-16042/CA/NCI NIH HHS/United States
- AI-28697/AI/NIAID NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- P50 CA90388/CA/NCI NIH HHS/United States
- U01 CA152751/CA/NCI NIH HHS/United States
- P50 CA090388/CA/NCI NIH HHS/United States
- P30 CA016042/CA/NCI NIH HHS/United States
- P30 AI028697/AI/NIAID NIH HHS/United States
- I01 BX000359/BX/BLRD VA/United States
- K23 CA149079/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials