Inhibition of dengue and chikungunya virus infections by RIG-I-mediated type I interferon-independent stimulation of the innate antiviral response - PubMed (original) (raw)
Inhibition of dengue and chikungunya virus infections by RIG-I-mediated type I interferon-independent stimulation of the innate antiviral response
David Olagnier et al. J Virol. 2014 Apr.
Abstract
RIG-I is a cytosolic sensor critically involved in the activation of the innate immune response to RNA virus infection. In the present study, we evaluated the inhibitory effect of a RIG-I agonist on the replication of two emerging arthropod-borne viral pathogens, dengue virus (DENV) and chikungunya virus (CHIKV), for which no therapeutic options currently exist. We demonstrate that when a low, noncytotoxic dose of an optimized 5'triphosphorylated RNA (5'pppRNA) molecule was administered, RIG-I stimulation generated a robust antiviral response against these two viruses. Strikingly, 5'pppRNA treatment before or after challenge with DENV or CHIKV provided protection against infection. In primary human monocytes and monocyte-derived dendritic cells, the RIG-I agonist blocked both primary infection and antibody-dependent enhancement of DENV infection. The protective response against DENV and CHIKV induced by 5'pppRNA was dependent on an intact RIG-I/MAVS/TBK1/IRF3 axis and was largely independent of the type I IFN response. Altogether, this in vitro analysis of the antiviral efficacy of 5'pppRNA highlights the therapeutic potential of RIG-I agonists against emerging viruses such as DENV and CHIKV.
Importance: DENV and CHIKV are two reemerging mosquito-borne viruses for which no therapeutic options currently exist. Both viruses overlap geographically in tropical regions of the world, produce similar fever-like symptoms, and are difficult to diagnose. This study investigated the inhibitory effect of a RIG-I agonist on the replication of these two viruses. RIG-I stimulation using 5'pppRNA before or after DENV or CHIKV infection generated a protective antiviral response against both pathogens in immune and nonimmune cells; interestingly, the protective response against the viruses was largely independent of the classical type I interferon response. The antiviral efficacy of 5'pppRNA highlights the therapeutic potential of RIG-I agonists against emerging viruses such as DENV and CHIKV.
Figures
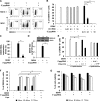
FIG 1
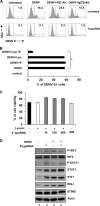
Pretreatment with 5′pppRNA inhibits DENV replication in vitro. (A and B) A549 cells were pretreated with various concentrations of 5′pppRNA (0.01 to 10 ng/ml) or control (Ctrl) RNA lacking the 5′ppp at the same concentrations for 24 h prior to DENV challenge. The percentage of DENV-infected cells was determined by intracellular staining (ICS) of DENV E protein expression using flow cytometry. Data are from two independent experiments performed in triplicate and represent the means ± SEM. *, P < 0.05. FSC, forward scatter. (C and D) A549 cells were pretreated with 5′pppRNA (1 ng/ml) for 24 h prior to DENV challenge (MOI, 0.1). DENV RNA level (C), viral titers (D), and DENV E protein expression level (D) were determined by RT-qPCR, plaque assay, and Western blotting, respectively. Error bars represent SEM from three independent samples. *, P < 0.05. One representative DENV E protein Western blot out of three independent triplicates is shown. (E) A549 cells were transfected using Lipofectamine (Lipo.) RNAiMax with increasing concentrations of 5′pppRNA and poly(I·C) (0.1 to 1 ng/ml) or treated with the same dsRNA sequences (5,000 ng/ml) in the absence of transfection reagent. Cells were then challenged with DENV (MOI, 1), and the percentage of infected cells was determined by FACS 24 h after infection. Data are the means ± SEM from two independent experiments performed in triplicate. *, P < 0.05. (F and G) The percentage of A549 DENV-infected cells and cell viability were assessed by flow cytometry and determined at 24 h (black bars), 48 h (gray bars), and 72 h (white bars) after DENV challenge (MOI, 0.01). Cells were pretreated with 5′pppRNA (1 ng/ml) for 24 h before DENV challenge. Data are the means ± SEM from a representative experiment performed in triplicate. *, P < 0.05.
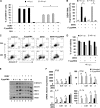
FIG 2
Postinfection treatment with 5′pppRNA inhibits de novo DENV infection. (A) A549 cells were treated with 5′pppRNA (1 ng/ml) 4 h (black bars) or 8 h (gray bars) following DENV challenge (MOI, 0.01). The percentage of DENV-infected cells was determined by intracellular staining (ICS) of DENV E protein expression using flow cytometry at 48 h after infection. Data represent the means ± SEM from a representative experiment performed in triplicate. *, P < 0.05. (B) DENV RNA levels were determined by RT-qPCR (48 h after infection) on A549 cells treated with 5′pppRNA (1 ng/ml) 4 h (black bars) and 8 h (gray bars) after infection. *, P < 0.05. (C and D) Cell viability of A549 cells was measured by flow cytometry 24 h (black bars) and 48 h (gray bars) after infection. Cells were treated with 5′pppRNA 4 h after DENV infection. Data are the means ± SEM from a representative experiment performed in triplicate. (E) A549 cells were challenged with DENV (MOI, 0.1) for 4 h and transfected with 5′pppRNA (0.1 to 10 ng/ml) and incubated for an additional 20 h. Whole-cell extracts (WCEs) were prepared and subjected to immunoblot analysis 24 h postinfection. Data are from one representative experiment. (F) A549 cells were infected with DENV at different MOI and were transfected with 5′pppRNA (1 ng/ml) 4 h after infection. The expression level of genes was determined by RT-qPCR 24 h after DENV challenge. Data are the means ± SEM from a representative experiment performed in triplicate. *, P < 0.05.
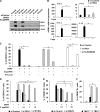
FIG 3
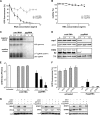
5′pppRNA inhibits DENV infection in vitro in a RIG-I/MAVS/TBK1-dependent manner. (A) A549 cells were transfected with control or RIG-I siRNA (10 or 30 pmol), and 48 h later they were treated with 5′pppRNA (10 ng/ml) for 24 h. Expression of IFIT1, RIG-I, and β-actin was evaluated by Western blotting. RIG-I knockdown and impairment of the 5′ppp-induced immune response is representative of at least 3 independent experiments. (B) A549 cells were transfected with control siRNA or RIG-I siRNA (30 pmol), and 48 h later they were treated with 5′pppRNA (10 ng/ml) for 24 h. mRNA expression level of IFN-β, IFN-α, TNF-α, and IL-29 was evaluated by RT-qPCR. Data are from a representative experiment performed in triplicate and show the means ± SEM. *, P < 0.05. (C) A549 cells were transfected with control (black bars), RIG-I (gray bars), or a combination of TLR3/MDA5 (white bars) siRNA (30 pmol each), and 48 h later they were treated with 5′pppRNA (10 ng/ml) or poly(I·C) (1 ng/ml). Cells were then infected with DENV (MOI, 0.5), and at 24 h p.i. the percentage of infected cells was assessed by intracellular staining of DENV E protein using flow cytometry. Data are from a representative experiment performed in triplicate and show the means ± SEM. *, P < 0.05. (D and E) A549 cells were treated with 5′pppRNA (0.1 to 10 ng/ml) for 24 h 2 days after transfection with 30 pmol of control (black bars), RIG-I (gray bars), or STING (white bars) siRNA (D) or with 30 pmol of control (black bars) or MAVS (gray bars) siRNA (E). Cells were then challenged with DENV (MOI, 0.1) for 24 h. The percentage of DENV-infected cells was determined by intracellular staining of DENV E protein and flow cytometry 24 h after infection. Data are the means ± SEM from a representative experiment performed in triplicate. *, P < 0.05. (F) TBK1+/+ (black bars) and TBK1−/− (gray bars) MEF cells were treated with 10 ng/ml of 5′pppRNA 24 h before DENV challenge at an MOI of 5. The percentage of DENV-infected cells was evaluated by flow cytometry. Data are the means ± SEM of a representative experiment performed in triplicate. *, P < 0.05.
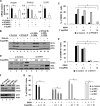
FIG 4
5′pppRNA-induced antiviral effect is IRF3 dependent but IFNAR and STAT1 independent. (A) A549 cells were transfected with control, IFN-α/βR α chain (IFNAR1), IFN-α/βR β chain (IFNAR2), or IL-28R siRNA, and 48 h later mRNA levels of IFNAR1, IFNAR2, and IL-28R were evaluated by RT-qPCR. Data are from a representative experiment performed in triplicate. *, P < 0.05 (B) A549 cells were transfected with the control siRNA, IFN-α/βR or IL-28R siRNA, or a combination of both. After 48 h, cells were treated with 5′pppRNA (10 ng/ml) or IFN-α2b (100 UI/ml) for 24 h. Expression of IFIT1, RIG-I, and β-actin was evaluated by Western blotting. The evaluation of 5′ppp-induced immune response by Western blotting in the absence of type I IFN receptor, representative of three independent experiments, and in the absence of type III IFN receptor, representative of one experiment. (C) After siRNA knockdown of IFN-α/βR as described for panel B, cells were treated with increasing concentrations of 5′pppRNA (0.1 to 10 ng/ml) and then infected with DENV (MOI, 0.1). The percentage of DENV-infected cells was evaluated by flow cytometry. Data are the means ± SEM of a representative experiment performed in triplicate. *, P < 0.05. (D) A549 cells were transfected with control and STAT1 siRNA, and 48 h later they were treated with 5′pppRNA (0.01 to 1 ng/ml) for 24 h. Expression of STAT1, IFIT1, and β-actin was evaluated by Western blotting. The induction of 5′ppp-induced immune response in the absence of STAT is representative of two independent experiments. (E) A549 cells were transfected with control or STAT1 siRNA and incubated for 48 h. Cells were treated with increasing concentrations of 5′pppRNA (0.1 to 10 ng/ml) and then infected with DENV (MOI, 0.1). The percentage of DENV-infected cells was evaluated by flow cytometry. Data are the means ± SEM from a representative experiment performed in triplicate. *, P < 0.05. (F) A549 cells were transfected with control, IRF1, IRF3, or IRF7 siRNA for 48 h, and the protein expression level of these transcription factors was evaluated by Western blotting. This panel is representative of one experiment. (G) A549 cells were transfected with control IRF1, IRF3, or IRF7 and then treated as described for panel E. The percentage of DENV-infected cells was evaluated by flow cytometry. Data are the means ± SEM from a representative experiment performed in triplicate. *, P < 0.05.
FIG 5
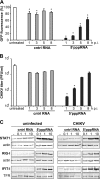
5′pppRNA treatment protects human myeloid cells from primary and ADE DENV infection. (A) Negatively selected monocytes were challenged with DENV (MOI, 20) in the presence or absence of the enhancing antibody 4G2 (0.5 μg/ml) for 4 h. They were subsequently transfected with 5′pppRNA (100 ng/ml) using Lyovec and incubated for 20 h. An IgG2a antibody (0.5 μg/ml) served as a negative control. The percentage of DENV-infected cells was determined by flow cytometry 24 h after infection. (B) CD14− MDDCs were challenged with DENV (MOI, 10) for 4 h, followed by transfection with 5′pppRNA (100 ng/ml) and incubation for an additional 20 h. Data represent the means ± SEM of an experiment performed in triplicate. *, P < 0.05. (C) Cell viability was assessed by flow cytometry on CD14− MDDC and determined 24 h after 5′pppRNA treatment (10 to 500 ng/ml) in the presence of Lyovec. Data are the means ± SEM of a representative experiment performed in triplicate. (D) CD14− MDDCs were challenged with DENV (MOI, 10) for 4 h and then were treated with 5′pppRNA (100 ng/ml) for an additional 20 h. WCEs were resolved by SDS-PAGE and analyzed by immunoblotting for phospo-IRF3, IRF3, phospho-STAT1, STAT1, IFIT1, RIG-I, STING, and β-actin. Results are from one representative experiment that was repeated once.
FIG 6
Treatment with 5′pppRNA inhibits CHIKV replication in a RIG-I-dependent manner. (A) MRC-5 cells were treated with 0.015 to 4 ng/ml of control RNA or 5′pppRNA from 1 h prior to infection to 24 h postinfection with CHIKV LS3-GFP (MOI, 0.1). At 24 h p.i., cells were fixed and EGFP reporter gene expression was quantified. *, P < 0.05. cntrl, control. (B) To assess potential cytotoxicity, MRC-5 cell viability was measured 24 h posttransfection of 5′pppRNA or control RNA lacking the 5′ triphosphate. Data are represented as the means ± SEM from a representative experiment performed in quadruplicate. (C) The intracellular accumulation of CHIKV positive- and negative-strand RNA was determined by in-gel hybridization of RNA isolated from MRC-5 cells that were treated with 5′pppRNA (0.1 to 10 ng/ml) 1 h prior to infection (MOI, 0.1). (D) CHIKV E2, E3E2, and nsP1 protein expression was assessed by Western blotting of lysates of MRC-5 cells that were treated with various concentrations of control RNA or 5′pppRNA 1 h prior to infection with CHIKV. Data are representative of at least two independent experiments. (E) The effect of 5′pppRNA and control RNA treatment on CHIKV progeny titers as assessed by plaque assay. (F) siRNA-transfected MRC-5 cells were either left untreated or were transfected with 5′pppRNA, after which they were infected with CHIKV LS3-GFP (MOI, 0.1). CHIKV-driven EGFP reporter gene expression was measured at 24 h p.i. and was normalized to the expression level in CHIKV-infected cells that had been transfected with a nontargeting scrambled siRNA (scr). *, P < 0.05. (G) MRC-5 cells were transfected with 10 pmol of scrambled siRNA (siScr) or siRNA targeting RIG-I, STAT1, or STING 48 h prior to treatment with 1 ng/ml of 5′pppRNA. Expression levels of RIG-I, STAT1, STING, and IFIT1 were monitored by Western blotting. Cyclophilin A or B was used as a loading control. Data are representative of at least two independent experiments.
FIG 7
Postinfection treatment with 5′pppRNA inhibits CHIKV replication in an addition-dependent manner. MRC-5 cells were infected with CHIKV LS3-GFP at an MOI of 0.1, and at the indicated time points postinfection they were transfected with 1 ng/ml 5′pppRNA or control RNA. (A) Cells were fixed at 24 h p.i., and EGFP reporter gene expression was quantified and normalized to that in untreated cells. *, P < 0.05. (B) CHIKV progeny titers 24 h p.i. and after 5′pppRNA or control RNA treatment were determined by plaque assay. (C) MRC-5 cells were transfected with 0.1, 1, or 10 ng/ml 5′pppRNA or control RNA 1 h prior to infection with CHIKV LS3-GFP (MOI, 0.1). At 24 h p.i., cell lysates were prepared and STAT1, RIG-I, and IFIT-I protein levels were determined by Western blotting. Actin or the transferrin receptor were used as loading controls. Data are representative of at least two independent experiments.
Similar articles
- Sequence-Specific Modifications Enhance the Broad-Spectrum Antiviral Response Activated by RIG-I Agonists.
Chiang C, Beljanski V, Yin K, Olagnier D, Ben Yebdri F, Steel C, Goulet ML, DeFilippis VR, Streblow DN, Haddad EK, Trautmann L, Ross T, Lin R, Hiscott J. Chiang C, et al. J Virol. 2015 Aug;89(15):8011-25. doi: 10.1128/JVI.00845-15. Epub 2015 May 27. J Virol. 2015. PMID: 26018150 Free PMC article. - Systems analysis of a RIG-I agonist inducing broad spectrum inhibition of virus infectivity.
Goulet ML, Olagnier D, Xu Z, Paz S, Belgnaoui SM, Lafferty EI, Janelle V, Arguello M, Paquet M, Ghneim K, Richards S, Smith A, Wilkinson P, Cameron M, Kalinke U, Qureshi S, Lamarre A, Haddad EK, Sekaly RP, Peri S, Balachandran S, Lin R, Hiscott J. Goulet ML, et al. PLoS Pathog. 2013;9(4):e1003298. doi: 10.1371/journal.ppat.1003298. Epub 2013 Apr 25. PLoS Pathog. 2013. PMID: 23633948 Free PMC article. - Dengue Virus Subverts Host Innate Immunity by Targeting Adaptor Protein MAVS.
He Z, Zhu X, Wen W, Yuan J, Hu Y, Chen J, An S, Dong X, Lin C, Yu J, Wu J, Yang Y, Cai J, Li J, Li M. He Z, et al. J Virol. 2016 Jul 27;90(16):7219-7230. doi: 10.1128/JVI.00221-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27252539 Free PMC article. - How Dengue Virus Circumvents Innate Immunity.
Kao YT, Lai MMC, Yu CY. Kao YT, et al. Front Immunol. 2018 Dec 4;9:2860. doi: 10.3389/fimmu.2018.02860. eCollection 2018. Front Immunol. 2018. PMID: 30564245 Free PMC article. Review. - The worldwide seroprevalence of DENV, CHIKV and ZIKV infection: A systematic review and meta-analysis.
Li Z, Wang J, Cheng X, Hu H, Guo C, Huang J, Chen Z, Lu J. Li Z, et al. PLoS Negl Trop Dis. 2021 Apr 28;15(4):e0009337. doi: 10.1371/journal.pntd.0009337. eCollection 2021 Apr. PLoS Negl Trop Dis. 2021. PMID: 33909610 Free PMC article.
Cited by
- RIG-I Activation by a Designer Short RNA Ligand Protects Human Immune Cells against Dengue Virus Infection without Causing Cytotoxicity.
Ho V, Yong HY, Chevrier M, Narang V, Lum J, Toh YX, Lee B, Chen J, Tan EY, Luo D, Fink K. Ho V, et al. J Virol. 2019 Jun 28;93(14):e00102-19. doi: 10.1128/JVI.00102-19. Print 2019 Jul 15. J Virol. 2019. PMID: 31043531 Free PMC article. - Immune Response to Dengue and Zika.
Ngono AE, Shresta S. Ngono AE, et al. Annu Rev Immunol. 2018 Apr 26;36:279-308. doi: 10.1146/annurev-immunol-042617-053142. Epub 2018 Jan 18. Annu Rev Immunol. 2018. PMID: 29345964 Free PMC article. Review. - NOD2 Responds to Dengue Virus Type 2 Infection in Macrophage-like Cells Interacting with MAVS Adaptor and Affecting IFN-α Production and Virus Titers.
Domínguez-Martínez DA, Pérez-Flores MS, Núñez-Avellaneda D, Torres-Flores JM, León-Avila G, García-Pérez BE, Salazar MI. Domínguez-Martínez DA, et al. Pathogens. 2024 Apr 10;13(4):306. doi: 10.3390/pathogens13040306. Pathogens. 2024. PMID: 38668261 Free PMC article. - Advances in antiviral strategies targeting mosquito-borne viruses: cellular, viral, and immune-related approaches.
Khan A, Zakirullah, Wahab S, Hong ST. Khan A, et al. Virol J. 2025 Feb 4;22(1):26. doi: 10.1186/s12985-025-02622-z. Virol J. 2025. PMID: 39905499 Free PMC article. Review. - Sequence-Specific Modifications Enhance the Broad-Spectrum Antiviral Response Activated by RIG-I Agonists.
Chiang C, Beljanski V, Yin K, Olagnier D, Ben Yebdri F, Steel C, Goulet ML, DeFilippis VR, Streblow DN, Haddad EK, Trautmann L, Ross T, Lin R, Hiscott J. Chiang C, et al. J Virol. 2015 Aug;89(15):8011-25. doi: 10.1128/JVI.00845-15. Epub 2015 May 27. J Virol. 2015. PMID: 26018150 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous