In vitro wound healing potential and identification of bioactive compounds from Moringa oleifera Lam - PubMed (original) (raw)
In vitro wound healing potential and identification of bioactive compounds from Moringa oleifera Lam
Abubakar Amali Muhammad et al. Biomed Res Int. 2013.
Expression of concern in
- Expression of Concern on "In Vitro Wound Healing Potential and Identification of Bioactive Compounds from Moringa oleifera Lam".
International BR. International BR. Biomed Res Int. 2020 Nov 29;2020:8687291. doi: 10.1155/2020/8687291. eCollection 2020. Biomed Res Int. 2020. PMID: 33313324 Free PMC article. No abstract available.
Abstract
Moringa oleifera Lam. (M. oleifera) from the monogeneric family Moringaceae is found in tropical and subtropical countries. The present study was aimed at exploring the in vitro wound healing potential of M. oleifera and identification of active compounds that may be responsible for its wound healing action. The study included cell viability, proliferation, and wound scratch test assays. Different solvent crude extracts were screened, and the most active crude extract was further subjected to differential bioguided fractionation. Fractions were also screened and most active aqueous fraction was finally obtained for further investigation. HPLC and LC-MS/MS analysis were used for identification and confirmation of bioactive compounds. The results of our study demonstrated that aqueous fraction of M. oleifera significantly enhanced proliferation and viability as well as migration of human dermal fibroblast (HDF) cells compared to the untreated control and other fractions. The HPLC and LC-MS/MS studies revealed kaempferol and quercetin compounds in the crude methanolic extract and a major bioactive compound Vicenin-2 was identified in the bioactive aqueous fraction which was confirmed with standard Vicenin-2 using HPLC and UV spectroscopic methods. These findings suggest that bioactive fraction of M. oleifera containing Vicenin-2 compound may enhance faster wound healing in vitro.
Figures
Figure 1
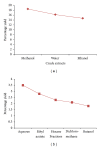
Percentage of yield of crude extracts and fractions of M. oleifera obtained following complete extraction of M. oleifera leaves. DCM: dichloromethane; EA: ethyl acetate. Values were obtained per 100 g of powdered leaves sample for the crude extracts and 10 g of dried methanolic extract for the aqueous fraction.
Figure 2
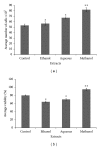
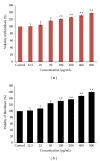
Effect of different crude extracts of M. oleifera on cell count and viability of human dermal fibroblast (HDF) administered at 12.5 _μ_g/mL. The data were expressed as mean ± SD of triplicate values. ANOVA was used for statistical analysis. **P < 0.05 (methanolic extract treated versus other solvent extracts and control).
Figure 3
Effects of different fractions of M. oleifera on cell count and percentage viability of HDF administered at 25 _μ_g/mL of each fraction and readings were taken after 72 hrs. Percentage viability was estimated using standard formula as % cell viability = total viable cells/total cells (dead + viable) × 100. Data were expressed as mean ± SD of triplicate values. **P < 0.05 (methanolic extract versus other solvent extracts and control).
Figure 4
Digital image showing the effect of different fractions of M. oleifera on human dermal fibroblast migration in a wound scratch test assay: (a) (i): control without treatment; (ii): methanol; (iii): ethanol; (iv): aqueous extracts; (b) (i): control; (ii): n-hexane; (iii): dichloromethane; (iv): ethyl acetate; (v): n-butanol; (vi): aqueous fractions. A confluent monolayer of human dermal fibroblast (HDF) was scratched using a sterilised 200 _μ_L pipette tip. Different fractions were applied as treatments to the wounded (open gap) and fibroblast media served as control. Migration of fibroblast cells were captured and measured using light microscope attached to a digital camera. Magnification (4x).
Figure 5
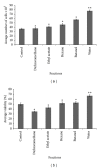
Effect of different crude extracts and fractions of M. oleifera on human dermal fibroblast in a wound scratch test. The enhanced migratory HDF cells that completely closed the gap created after 72 hrs were seen in methanolic crude extract and aqueous fraction compared to other solvent extracts. Values are expressed as mean ± SD of triplicate determinations. **P < 0.05 (methanolic extract versus control and other extracts in (a)). **P < 0.05 (aqueous fraction versus control and other fractions in (b)).
Figure 6
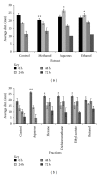
Effects of aqueous fraction treated human dermal fibroblasts (HDF) on cell proliferation and viability of various concentrations at 48 (a) and 72 hrs (b), respectively. The value from baseline control group was set at 100%. The proliferation activity was estimated by MTT assay and calculated by comparing the values from the aqueous fraction treated group with that of control group. Data were expressed as mean ± SD of triplicate values. **P < 0.05 (aqueous fraction treated groups versus control group).
Figure 7
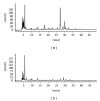
HPLC-DAD chromatogram of (a) methanolic crude extract and (b) aqueous fraction of M. oleifera. The peaks represent general profiling of compounds and the pattern of distribution showed a combination of polar, intermediate polar, and nonpolar compounds as represented in methanolic crude extract, while only polar region was observed in aqueous fraction.
Figure 8
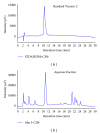
HPLC-DAD chromatogram for reference standard Vicenin-2 (a) and aqueous fraction sample of M. oleifera (b). Both peaks have similar retention time of about 11 mins indicating that our aqueous fraction sample contains Vicenin-2 as the major active compound.
Similar articles
- Evaluation of wound healing properties of bioactive aqueous fraction from Moringa oleifera Lam on experimentally induced diabetic animal model.
Muhammad AA, Arulselvan P, Cheah PS, Abas F, Fakurazi S. Muhammad AA, et al. Drug Des Devel Ther. 2016 May 24;10:1715-30. doi: 10.2147/DDDT.S96968. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27307703 Free PMC article. - Development and formulation of Moringa oleifera standardised leaf extract film dressing for wound healing application.
Chin CY, Jalil J, Ng PY, Ng SF. Chin CY, et al. J Ethnopharmacol. 2018 Feb 15;212:188-199. doi: 10.1016/j.jep.2017.10.016. Epub 2017 Nov 5. J Ethnopharmacol. 2018. PMID: 29080829 - Self-microemulsifying drug delivery systems of Moringa oleifera extract for enhanced dissolution of kaempferol and quercetin.
Sermkaew N, Plyduang T. Sermkaew N, et al. Acta Pharm. 2020 Mar 1;70(1):77-88. doi: 10.2478/acph-2020-0012. Acta Pharm. 2020. PMID: 31677372 - Scoping Review: Evaluation of Moringa oleifera (Lam.) for Potential Wound Healing in In Vivo Studies.
Mohammad Shafie N, Raja Shahriman Shah RNI, Krishnan P, Abdul Haleem N, Tan TYC. Mohammad Shafie N, et al. Molecules. 2022 Aug 28;27(17):5541. doi: 10.3390/molecules27175541. Molecules. 2022. PMID: 36080308 Free PMC article. Review. - Bioactive components and anti-diabetic properties of Moringa oleifera Lam.
Wang F, Bao Y, Zhang C, Zhan L, Khan W, Siddiqua S, Ahmad S, Capanoglu E, Skalicka-Woźniak K, Zou L, Simal-Gandara J, Cao H, Weng Z, Shen X, Xiao J. Wang F, et al. Crit Rev Food Sci Nutr. 2022;62(14):3873-3897. doi: 10.1080/10408398.2020.1870099. Epub 2021 Jan 6. Crit Rev Food Sci Nutr. 2022. PMID: 33401950 Review.
Cited by
- Histological Assessment of Palatal Donor Site Wound Healing after Application of Moringa oleifera Lamarck Leaf Extract in Rats.
Amaliya A, Muhaimina RK, Susanto A, Sutjiatmo AB. Amaliya A, et al. Eur J Dent. 2019 May;13(2):248-254. doi: 10.1055/s-0039-1695065. Epub 2019 Sep 11. Eur J Dent. 2019. PMID: 31509874 Free PMC article. - An Investigation on the In Vitro Wound Healing Activity and Phytochemical Composition of Hypericum pseudolaeve N. Robson Growing in Turkey.
Kaptaner İğci B, Aytaç Z. Kaptaner İğci B, et al. Turk J Pharm Sci. 2020 Dec 23;17(6):610-619. doi: 10.4274/tjps.galenos.2019.80037. Turk J Pharm Sci. 2020. PMID: 33389950 Free PMC article. - Wound healing properties of ethyl acetate fraction of Moringa oleifera in normal human dermal fibroblasts.
Gothai S, Arulselvan P, Tan WS, Fakurazi S. Gothai S, et al. J Intercult Ethnopharmacol. 2016 Feb 8;5(1):1-6. doi: 10.5455/jice.20160201055629. eCollection 2016 Jan-Feb. J Intercult Ethnopharmacol. 2016. PMID: 27069722 Free PMC article. - Mechanistic Wound Healing and Antioxidant Potential of Moringa oleifera Seeds Extract Supported by Metabolic Profiling, In Silico Network Design, Molecular Docking, and In Vivo Studies.
Shady NH, Mostafa NM, Fayez S, Abdel-Rahman IM, Maher SA, Zayed A, Saber EA, Khowdiary MM, Elrehany MA, Alzubaidi MA, Altemani FH, Shawky AM, Abdelmohsen UR. Shady NH, et al. Antioxidants (Basel). 2022 Sep 1;11(9):1743. doi: 10.3390/antiox11091743. Antioxidants (Basel). 2022. PMID: 36139817 Free PMC article. - Topical Wound Healing Activity of Myricetin Isolated from Tecomaria capensis v. aurea.
Elshamy AI, Ammar NM, Hassan HA, El-Kashak WA, Al-Rejaie SS, Abd-ElGawad AM, Farrag AH. Elshamy AI, et al. Molecules. 2020 Oct 22;25(21):4870. doi: 10.3390/molecules25214870. Molecules. 2020. PMID: 33105570 Free PMC article.
References
- Nagori BP, Solanki R. Role of medicinal plants in wound healing. Research Journal of Medicinal Plant. 2011;5(4):392–405.
- Tam JCW, Lau KM, Liu CL, et al. The in vivo and in vitro diabetic wound healing effects of a 2-herb formula and its mechanisms of action. Journal of Ethnopharmacology. 2011;134(3):831–838. - PubMed
- Nayak BS, Marshall MR, Isitor G. Wound healing potential of ethanolic extract of Kalanchoe pinnata lam. leaf-a preliminary study. Indian Journal of Experimental Biology. 2010;48(6):572–576. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources