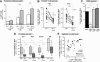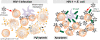Microbial exposure alters HIV-1-induced mucosal CD4+ T cell death pathways Ex vivo - PubMed (original) (raw)
Microbial exposure alters HIV-1-induced mucosal CD4+ T cell death pathways Ex vivo
Amanda K Steele et al. Retrovirology. 2014.
Abstract
Background: Early HIV-1 infection causes massive CD4+ T cell death in the gut and translocation of bacteria into the circulation. However, the programmed cell death (PCD) pathways used by HIV-1 to kill CD4+ T cells in the gut, and the impact of microbial exposure on T cell loss, remain unclear. Understanding mucosal HIV-1 triggered PCD could be advanced by an ex vivo system involving lamina propria mononuclear cells (LPMCs). We therefore modeled the interactions of gut LPMCs, CCR5-tropic HIV-1 and a commensal gut bacterial species, Escherichia coli. In this Lamina Propria Aggregate Culture (LPAC) model, LPMCs were infected with HIV-1BaL by spinoculation and cultured in the presence or absence of heat killed E.coli. CD4+ T cell numbers derived from flow cytometry and viable cell counts were reported relative to mock infection. Viable cells were identified by viability dye exclusion (AqVi), and intracellular HIV-1 Gag p24 protein was used to identify infected cells. Annexin V and AqVi were used to identify apoptotic versus necrotic cells. Caspase-1 and Caspase-3 activities were blocked using specific inhibitors YVAD and DEVD, respectively.
Results: CD4+ T cell depletion following HIV-1 infection was reproducibly observed by 6 days post infection (dpi). Depletion at 6 dpi strongly correlated with infection frequency at 4 dpi, was significantly blocked by Efavirenz treatment, and was primarily driven by p24-negative cells that were predominantly necrotic. HIV-1 infection significantly induced CD4+ T-cell intrinsic Caspase-1 activity, whereas Caspase-1 inhibition, but not Caspase-3 inhibition, significantly blocked CD4+ T cell depletion. Exposure to E.coli enhanced HIV-1 infection and CD4+ T depletion, and significantly increased the number of apoptotic p24+ cells. Notably, CD4+ T cell depletion in the presence of E.coli was partially blocked by Caspase-3, but not by Caspase-1 inhibition.
Conclusions: In the LPAC model, HIV-1 induced Caspase-1 mediated pyroptosis in bystander CD4+ T cells, but microbial exposure shifted the PCD mechanism toward apoptosis of productively infected T cells. These results suggest that mucosal CD4+ T cell death pathways may be altered in HIV-infected individuals after gut barrier function is compromised, with potential consequences for mucosal inflammation, viral dissemination and systemic immune activation.
Figures
Figure 1
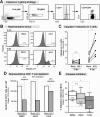
HIV-1 mediated cell death can be quantified in the LPAC model. (A) Lamina Propria Aggregate Culture (LPAC) model. Primary LPMCs from discarded surgical samples were infected with CCR5-tropic HIV-1Ba-L (80 ng p24 per 1 M LPMC), cultured for 1–6 days and harvested for flow cytometry. (B) Representative gating strategy. (C) The absolute number of CD4+ T cells per well in mock or HIV-1 infected conditions. N = 17 donors. Each symbol is a unique donor. Significant differences at each time point was determined by Wilcoxon matched pairs signed rank test. (D) To compare relative depletion between donors, CD4+ T cell survival was normalized to mock infection (100% survival). Each symbol is an individual donor. Horizontal solid lines reflect the median. Significance was determined using the Kruskal-Wallis test across timepoints. (E) Cell-specificity of HIV-1 mediated depletion in the LPAC model. The percent survival relative to mock infection at 6 dpi was determined for CD3+CD8- T cells (white), CD3+CD8+ T cells (light grey), and CD3-CD19+ B cells (dark grey). The black bar depicts 100% survival for each cell type in the mock infected condition, also indicated by the dashed horizontal line. Significance was determined using the Kruskal-Wallis test and Dunn’s multiple comparison test to compare selected pairs of columns. **p = 0.0015; ***p = 0.0003; ****p < 0.0001.
Figure 2
Relationship between HIV-1 infection and LP CD4+ T cell depletion. (A) Representative flow plots identifying p24+ cells using the gate established in mock infection (<1%). The net frequency of p24+CD4+ T cells (e.g.,%p24+ =%p24+ in HIV-1 minus %p24+ in Mock) is reported. (B) Spearman rank correlation analysis between the percentage of p24+ cells at 4 dpi and the level of LP CD4+ T cell depletion at 6 dpi. The best-fit line, Spearman r and the p value are indicated. (C) The percentage of p24+ cells was compared between ‘depleters’ and ‘non-depleters.’ Each point is a unique sample. The horizontal solid line indicates the median infection level. Statistical significance was determined by Mann–Whitney U test, *p = 0.03. (D-E) LPMCs were pre-treated with Efavirenz for 2 h prior to infection with HIV-1Ba-L or mock and re-dosed at 3 dpi. The net frequencies of p24+ cells (overall ANOVA p = 0.0008) were calculated for each donor at 6 dpi (Figure 2D). The percentage of CD4+ T cells surviving relative to mock infection in the presence of Efavirenz is shown (overall ANOVA p = 0.0394) (Figure 2E). The medians are shown and the dashed horizontal line indicates 100% survival relative to mock infection. Statistically significant differences were determined using the Friedman Test and Dunn’s Multiple Comparison test to compare between groups.
Figure 3
HIV-1 infection increases cell death markers in LP CD4+ T cells. (A) Representative flow plot of AnnexinV/AqVi staining. Note that in contrast to Figure 1B, there was no AqVi exclusion gate prior to identifying lymphocytes from the FSC/SSC profile. Cells expressing AnnexinV were considered committed to dying. (B) The percentage of CD3+CD8- T cells expressing either an apoptotic (left) or necrotic (right) profiles were compared between mock and HIV-1 infected conditions. Each symbol is a unique donor. The fold differences in median expression are shown. Significance was determined using the Wilcoxon matched-pairs signed rank test. (C) The numbers of p24+ and p24neg cells per well with an apoptotic or necrotic phenotype were calculated. Each symbol represents a unique donor. The median values are shown and the fold-change is indicated for significant differences. Significance was determined using the Wilcoxon matched-pairs signed rank test. (D) Apoptotic versus necrotic p24+ and p24neg cells as a percentage of the total number of LP CD3+CD8- T cells committed to death (n = 11). (E-F) Spearman rank correlation analysis between the number of AnnexinV+ (E) p24neg and (F) p24+ cells 4 dpi versus the 6 dpi depletion levels. The best-fit lines, Spearman r and p values are indicated. **, p = 0.009; ***, p = 0.001.
Figure 4
HIV-1 induced T cell Caspase-1 activity and death in non-productively infected LP CD4+ T cells. (A) Detection of active Caspase-1 in T cells. At 2 and 6 dpi, LPMCs were harvested and incubated for 30 min with the CaspaLux-1 Substrate (Oncoimmunin) then surface stained to allow for identification of CD3+CD8- T cells by flow cytometry. Cells were gated from a tight lymphocyte gate that excluded cellular debris. Cleaved CaspaLux-1-E1D2 was detected in CD3+CD8- T cells using the FITC channel. (B) Representative histograms of Caspalux-1-E1D2 staining. (C) HIV-1 induced Caspase-1 activity in LP CD4+ T cells. The fold change in median Caspase-1 activity is shown at 6 dpi. Significance was determined using the Wilcoxon matched-pairs signed rank test. (D). YVAD (Caspase-1; 25 μM), DEVD (Caspase-3; 25 μM) or a DMSO vehicle control were added at 0 and 2 dpi and CD4+ T cell survival was evaluated at 4 dpi. LP CD4+ T cell survival was normalized to parallel mock infections. The number of cells per well in a representative donor with mock (white bar) or HIV-1 (grey bar) infection with DMSO (left), DEVD (center) and YVAD (right). The percent depletion from mock are indicated for each condition. (E) The percent depletion from mock infected controls are shown in the presence of DMSO (white box), DEVD (light grey), and YVAD (dark grey) (n = 6). Box and whiskers indicate the median and range respectively. Significant differences in the percent depletion from the DMSO vehicle were determined using non-parametric repeated measures ANOVA. Dunn’s multiple comparison tests were used to compare selected pairs of columns as shown. Overall ANOVA, p = 0.0003. *p = 0.02–0.03.
Figure 5
Commensal E. coli enhance apoptosis in productively infected LP CD4+ T cells. (A) Commensal E. coli increases the frequency of productively infected cells. The percentage of p24+ cells in the presence of a 5:1 E. coli: LPMC ratio was quantified using intracellular p24 expression. Significance was determined using the Wilcoxon matched-pairs signed rank test. (B) LP CD4+ T cell survival was compared in the presence of absence of E. coli normalized to mock (100% survival). Significant differences in cell survival at 4 dpi or 6 dpi were determined using the Wilcoxon matched-pairs signed rank test. (C) Cell-specificity of HIV-1 mediated depletion in the LPAC model. The percent survival relative to mock infection at 6 dpi was determined for CD3+CD8- T cells (white), CD3+CD8+ T cells (light grey), and CD3-CD19+ B cells (dark grey). The black bar depicts 100% survival for each cell type in the mock infected condition, also indicated by the dashed horizontal line. Significance was determined using the Kruskal-Wallis test and Dunn’s multiple comparison tests to compare selected pairs of columns. (D) Th Subset survival was determined following stimulation with PMA/Ionomycin. Viable cell counts were obtained prior to PMA/Ionomycin and multipled by the percentage of CD3+CD8- T cells expressing cytokine. Survival was determined normalized to mock infection for all subsets. Box and whiskers indicate the median and range respectively. Significant differences in cell survival for each subset were determined using the Wilcoxon matched-pairs signed rank test. (E) Spearman rank correlation analysis between the percentage of p24+ cells at 4 dpi and the level of LP CD4+ T cell depletion at 6 dpi. The best-fit line, Spearman r and the p value are indicated. *p = <0.05; ***p = 0.0005; ****p < 0.0001.
Figure 6
E. coli alters caspase-dependent LP CD4+ T cell death. (A and B) Impact of E.coli exposure on apoptotic and necrotic phenotypes in the LPAC model. The absolute numbers of p24+ cells that were either apoptotic (left) or necrotic (right) were determined as in Figure 3 for (A) p24+ and (B) p24neg cells. Each symbol represents a unique donor. Significance was determined using the Wilcoxon matched-pairs signed rank test within each phenotype. (C) Apoptotic versus necrotic p24+ or p24neg cells as a percentage of the total LP CD3+CD8- T cells committed to death (n = 11). Compare to Figure 4B. (D) Impact of E.coli on cell death markers in uninfected LPMCs. The percentage of apoptotic or necrotic mock-infected cells were compared with or without E.coli. The horizontal lines indicate the median values and the fold-differences in cell death marker expression. Significance was determined using the Wilcoxon matched-pairs signed rank test within each phenotype. (E) The percent depletion from mock infection in the presence of YVAD and DEVD (n = 7) as described in Figure 5 in the presence of E.coli. Overall ANOVA, p = 0.008. *p = < 0.05 **, p = 0.007; ***, p = 0.001.
Figure 7
Biphasic model of LP CD4+ T cell death during acute HIV-1 infection. We theorize that in ‘early’ acute infection, prior to extensive microbial translation, pyroptotic death in bystander p24neg cells could account for the majority of LP CD4+ T cell depletion. Inflammatory mediators released during pyroptosis may contribute to epithelial barrier break down. During ‘late’ acute HIV-1 infection, T cells and APCs exposed to commensal bacteria results in enhanced HIV-1 replication, and a shift in the LP CD4+ T cell death pathway to apoptosis. The loss of Th17 cells in particular could further exacerbate epithelial barrier dysfunction and contribute to a cycle of microbial entry, T cell activation, infection and death. Increased apoptotsis from productively infected cells could also contribute to the release of apoptotic microparticles into the periphery, which others have shown could reduce the potency of early adaptive immune responses against HIV-1.
Similar articles
- Enhancement of HIV-1 infection and intestinal CD4+ T cell depletion ex vivo by gut microbes altered during chronic HIV-1 infection.
Dillon SM, Lee EJ, Donovan AM, Guo K, Harper MS, Frank DN, McCarter MD, Santiago ML, Wilson CC. Dillon SM, et al. Retrovirology. 2016 Jan 14;13:5. doi: 10.1186/s12977-016-0237-1. Retrovirology. 2016. PMID: 26762145 Free PMC article. - HIV-1 infection of human intestinal lamina propria CD4+ T cells in vitro is enhanced by exposure to commensal Escherichia coli.
Dillon SM, Manuzak JA, Leone AK, Lee EJ, Rogers LM, McCarter MD, Wilson CC. Dillon SM, et al. J Immunol. 2012 Jul 15;189(2):885-96. doi: 10.4049/jimmunol.1200681. Epub 2012 Jun 11. J Immunol. 2012. PMID: 22689879 Free PMC article. - A Matter of Life or Death: Productively Infected and Bystander CD4 T Cells in Early HIV Infection.
Cao D, Khanal S, Wang L, Li Z, Zhao J, Nguyen LN, Nguyen LNT, Dang X, Schank M, Thakuri BKC, Zhang J, Lu Z, Wu XY, Morrison ZD, El Gazzar M, Ning S, Moorman JP, Yao ZQ. Cao D, et al. Front Immunol. 2021 Feb 12;11:626431. doi: 10.3389/fimmu.2020.626431. eCollection 2020. Front Immunol. 2021. PMID: 33643305 Free PMC article. - Mucosal immune dysfunction in AIDS pathogenesis.
Paiardini M, Frank I, Pandrea I, Apetrei C, Silvestri G. Paiardini M, et al. AIDS Rev. 2008 Jan-Mar;10(1):36-46. AIDS Rev. 2008. PMID: 18385779 Review. - Mucosal immunity and HIV-1 infection: applications for mucosal AIDS vaccine development.
Belyakov IM, Ahlers JD. Belyakov IM, et al. Curr Top Microbiol Immunol. 2012;354:157-79. doi: 10.1007/82_2010_119. Curr Top Microbiol Immunol. 2012. PMID: 21203884 Review.
Cited by
- Qualitative Differences Between the IFNα subtypes and IFNβ Influence Chronic Mucosal HIV-1 Pathogenesis.
Guo K, Shen G, Kibbie J, Gonzalez T, Dillon SM, Smith HA, Cooper EH, Lavender K, Hasenkrug KJ, Sutter K, Dittmer U, Kroehl M, Kechris K, Wilson CC, Santiago ML. Guo K, et al. PLoS Pathog. 2020 Oct 16;16(10):e1008986. doi: 10.1371/journal.ppat.1008986. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33064743 Free PMC article. - The gut microbiome and HIV-1 pathogenesis: a two-way street.
Dillon SM, Frank DN, Wilson CC. Dillon SM, et al. AIDS. 2016 Nov 28;30(18):2737-2751. doi: 10.1097/QAD.0000000000001289. AIDS. 2016. PMID: 27755100 Free PMC article. Review. - Intestinal Microbiota Dysbiosis Promotes Mucosal Barrier Damage and Immune Injury in HIV-Infected Patients.
Pan Z, Wu N, Jin C. Pan Z, et al. Can J Infect Dis Med Microbiol. 2023 Oct 28;2023:3080969. doi: 10.1155/2023/3080969. eCollection 2023. Can J Infect Dis Med Microbiol. 2023. PMID: 37927531 Free PMC article. Review. - Toll-Like Receptor 2 Ligation Enhances HIV-1 Replication in Activated CCR6+ CD4+ T Cells by Increasing Virus Entry and Establishing a More Permissive Environment to Infection.
Bolduc JF, Ouellet M, Hany L, Tremblay MJ. Bolduc JF, et al. J Virol. 2017 Jan 31;91(4):e01402-16. doi: 10.1128/JVI.01402-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27928019 Free PMC article. - Interferon-α Subtypes in an Ex Vivo Model of Acute HIV-1 Infection: Expression, Potency and Effector Mechanisms.
Harper MS, Guo K, Gibbert K, Lee EJ, Dillon SM, Barrett BS, McCarter MD, Hasenkrug KJ, Dittmer U, Wilson CC, Santiago ML. Harper MS, et al. PLoS Pathog. 2015 Nov 3;11(11):e1005254. doi: 10.1371/journal.ppat.1005254. eCollection 2015. PLoS Pathog. 2015. PMID: 26529416 Free PMC article.
References
- Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. - DOI - PMC - PubMed
- Sankaran S, Guadalupe M, Reay E, George MD, Flamm J, Prindiville T, Dandekar S. Gut mucosal T cell responses and gene expression correlate with protection against disease in long-term HIV-1-infected nonprogressors. Proc Natl Acad Sci U S A. 2005;102:9860–9865. doi: 10.1073/pnas.0503463102. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK088663/DK/NIDDK NIH HHS/United States
- R01 AI108404/AI/NIAID NIH HHS/United States
- T32 AI007447/AI/NIAID NIH HHS/United States
- 2T32AI007447-21/AI/NIAID NIH HHS/United States
- L30 AI084776/AI/NIAID NIH HHS/United States
- AI108404/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials