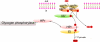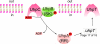The involvement of transport proteins in transcriptional and metabolic regulation - PubMed (original) (raw)
Review
The involvement of transport proteins in transcriptional and metabolic regulation
Ake Västermark et al. Curr Opin Microbiol. 2014 Apr.
Abstract
Transport proteins have sometimes gained secondary regulatory functions that influence gene expression and metabolism. These functions allow communication with the external world via mechanistically distinctive signal transduction pathways. In this brief review we focus on three transport systems in Escherichia coli that control and coordinate carbon, exogenous hexose-phosphate and phosphorous metabolism. The transport proteins that play central roles in these processes are: first, the phosphoenolpyruvate (PEP)-dependent phosphotransferase system (PTS), second, the glucose-6-phosphate receptor, UhpC, and third, the phosphate-specific transporter, PstSABC, respectively. While the PTS participates in multiple complex regulatory processes, three of which are discussed here, UhpC and the Pst transporters exemplify differing strategies.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
Fig. 1. PTS-mediated catabolite repression and inducer exclusion
Principal signal transduction pathways for carbon regulation in E. coli. A sugar such as glucose (Glc) is transported into the cell cytoplasm and concomitantly phosphorylated by the PTS, resulting in dephosphorylation of IIAGlc. Non-phosphorylated IIAGlc inhibits non-PTS sugar catabolic enzymes and transporters causing inducer exclusion. Phosphorylated IIAGlc, on the other hand, promotes cAMP formation by binding to adenylate cyclase and allosterically activating it. cAMP-driven transcription results when cAMP binds to CRP, a transcriptional activator. The rate-limiting step in the PTS phosphoryl transfer chain appears to be Enzyme I-catalyzed phosphorylation of HPr. ⊥, inhibition; →, activation.
Fig. 2. Regulation of glycogen breakdown by HPr of the PTS
HPr, the phosphate donor to PTS IIA proteins, becomes dephosphorylated when exogenous PTS sugars such as glucose are available. Dephospho-HPr then binds to glycogen phosphorylase, preventing glucose-1-phosphate release from glycogen. ⊥, inhibition.
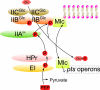
Fig. 3. Direct binding of a transcriptional repressor, Mlc, to the PTS Glucose Enzyme II
IIBCGlc in the unphosphorylated state (ie., when glucose is present) sequesters Mlc, preventing repression of pts genes. However, when glucose is absent, IIBCGlc is phosphorylated, causing release of Mlc so it represses expression of these genes. ⊥, repression.
Fig. 4. The uptake hexose phosphate (UHP) transport system
The Uhp system consists of four proteins: UhpA, B, C, and T. UhpC and UhpT are homologous members of the major facilitator superfamily, serving the function of receptor/transporter and transporter, respectively. UhpB and A are the sensor kinase and response regulator of the regulatory two component system, serving to convey the signal of glucose-6-phosphate (G6P) availability to upregulate the expression of the uhpT gene. The 2 TMS membrane domain of the UhpB protein senses when the substrate, G6P, for which UhpC is highly specific, is bound to its external surface. Then with G6P bound to UhpC, and with UhpC in direct contact with UhpB, UhpB utilizes ATP to autophosphorylate its central domain. Once UhpB is phosphorylated, UhpB can donate its phosphoryl group to UhpA, which binds to multiple sites between -80 and -32 upstream of the start site of the uhpT gene, functioning as an enhancer in the promoter region. →, transcriptional activation.
Fig 5. The phosphate-specific transporter, PstSABC, a transporter functioning in signal transduction
The phosphate (pho) regulon uses PstS, a periplasmic inorganic phosphate (Pi) binding receptor that serves as a constituent of the ABC transport complex, PstSABC. It also serves as a sensor of external Pi concentration. PstS senses the phosphate concentration in the periplasmic space, transmits a signal via PstABC and PhoU to the sensor kinase, PhoR, independently of the transport activity of PstSABC, thereby influencing expression of pho regulon genes. Therefore, the transporter is a sensor that senses extracellular phosphate to control gene expression. ⊥, inhibition; →, activation.
Similar articles
- Protein:Protein interactions in the cytoplasmic membrane apparently influencing sugar transport and phosphorylation activities of the e. coli phosphotransferase system.
Aboulwafa M, Zhang Z, Saier MH Jr. Aboulwafa M, et al. PLoS One. 2019 Nov 21;14(11):e0219332. doi: 10.1371/journal.pone.0219332. eCollection 2019. PLoS One. 2019. PMID: 31751341 Free PMC article. - Nutrient-scavenging stress response in an Escherichia coli strain lacking the phosphoenolpyruvate: carbohydrate phosphotransferase system, as explored by gene expression profile analysis.
Flores S, Flores N, de Anda R, González A, Escalante A, Sigala JC, Gosset G, Bolívar F. Flores S, et al. J Mol Microbiol Biotechnol. 2005;10(1):51-63. doi: 10.1159/000090348. J Mol Microbiol Biotechnol. 2005. PMID: 16491026 - Current knowledge of the Escherichia coli phosphoenolpyruvate-carbohydrate phosphotransferase system: peculiarities of regulation and impact on growth and product formation.
Escalante A, Salinas Cervantes A, Gosset G, Bolívar F. Escalante A, et al. Appl Microbiol Biotechnol. 2012 Jun;94(6):1483-94. doi: 10.1007/s00253-012-4101-5. Epub 2012 May 11. Appl Microbiol Biotechnol. 2012. PMID: 22573269 Review. - Inactivation of the PTS as a Strategy to Engineer the Production of Aromatic Metabolites in Escherichia coli.
Carmona SB, Moreno F, Bolívar F, Gosset G, Escalante A. Carmona SB, et al. J Mol Microbiol Biotechnol. 2015;25(2-3):195-208. doi: 10.1159/000380854. Epub 2015 Jul 9. J Mol Microbiol Biotechnol. 2015. PMID: 26159079 Review.
Cited by
- What bacteria want.
Galperin MY. Galperin MY. Environ Microbiol. 2018 Dec;20(12):4221-4229. doi: 10.1111/1462-2920.14398. Epub 2018 Oct 25. Environ Microbiol. 2018. PMID: 30187651 Free PMC article. Review. - Sequence- and Structure-Based Functional Annotation and Assessment of Metabolic Transporters in Aspergillus oryzae: A Representative Case Study.
Raethong N, Wong-Ekkabut J, Laoteng K, Vongsangnak W. Raethong N, et al. Biomed Res Int. 2016;2016:8124636. doi: 10.1155/2016/8124636. Epub 2016 May 4. Biomed Res Int. 2016. PMID: 27274991 Free PMC article. - Time to Stop Holding the Elevator: A New Piece of the Transport Protein Mechanism Puzzle.
Vastermark A, Saier MH Jr. Vastermark A, et al. Structure. 2016 Jun 7;24(6):845-6. doi: 10.1016/j.str.2016.05.010. Structure. 2016. PMID: 27276425 Free PMC article. - The Bacterial Phosphotransferase System: New Frontiers 50 Years after Its Discovery.
Saier MH Jr. Saier MH Jr. J Mol Microbiol Biotechnol. 2015;25(2-3):73-8. doi: 10.1159/000381215. Epub 2015 Jul 9. J Mol Microbiol Biotechnol. 2015. PMID: 26159069 Free PMC article. - Protein Activity Sensing in Bacteria in Regulating Metabolism and Motility.
Alvarado A, Behrens W, Josenhans C. Alvarado A, et al. Front Microbiol. 2020 Jan 17;10:3055. doi: 10.3389/fmicb.2019.03055. eCollection 2019. Front Microbiol. 2020. PMID: 32010106 Free PMC article. Review.
References
- Saier MH, Jr., Reizer J. The bacterial phosphotransferase system: new frontiers 30 years later. Molecular microbiology. 1994;13:755–764. [Summarizes the developments until 1994.] - PubMed
- Lee CA, Saier MH., Jr. Mannitol-specific enzyme II of the bacterial phosphotransferase system. III. The nucleotide sequence of the permease gene. The Journal of biological chemistry. 1983;258:10761–10767. - PubMed
- Fairbrother WJ, Gippert GP, Reizer J, Saier MH, Jr., Wright PE. Low resolution solution structure of the Bacillus subtilis glucose permease IIA domain derived from heteronuclear three-dimensional NMR spectroscopy. FEBS letters. 1992;296:148–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM077402/GM/NIGMS NIH HHS/United States
- U54 GM094610/GM/NIGMS NIH HHS/United States
- GM077402-05A1/GM/NIGMS NIH HHS/United States
- GM 094610-01/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous