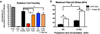3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration - PubMed (original) (raw)
3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration
Jason A Inzana et al. Biomaterials. 2014 Apr.
Abstract
Low temperature 3D printing of calcium phosphate scaffolds holds great promise for fabricating synthetic bone graft substitutes with enhanced performance over traditional techniques. Many design parameters, such as the binder solution properties, have yet to be optimized to ensure maximal biocompatibility and osteoconductivity with sufficient mechanical properties. This study tailored the phosphoric acid-based binder solution concentration to 8.75 wt% to maximize cytocompatibility and mechanical strength, with a supplementation of Tween 80 to improve printing. To further enhance the formulation, collagen was dissolved into the binder solution to fabricate collagen-calcium phosphate composites. Reducing the viscosity and surface tension through a physiologic heat treatment and Tween 80, respectively, enabled reliable thermal inkjet printing of the collagen solutions. Supplementing the binder solution with 1-2 wt% collagen significantly improved maximum flexural strength and cell viability. To assess the bone healing performance, we implanted 3D printed scaffolds into a critically sized murine femoral defect for 9 weeks. The implants were confirmed to be osteoconductive, with new bone growth incorporating the degrading scaffold materials. In conclusion, this study demonstrates optimization of material parameters for 3D printed calcium phosphate scaffolds and enhancement of material properties by volumetric collagen incorporation via inkjet printing.
Keywords: Bone regeneration; Calcium phosphate scaffold; Collagen; Three dimensional printing; Tissue engineering.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosure
The authors have no conflicts of interest and nothing to disclose.
Figures
Figure 1. Optimization of binder solution acidity to maximize cytocompatibility and mechanical strength
a) Using media extraction techniques with XTT assays after 24 hours of exposure to test material cytotoxicity according to ISO 10993, significant improvements in C3H/10T1/2 cell viability were observed when decreasing binder acidity from 12.5 wt% to 8.75 wt%. No significant improvements were observed when the acidity was further decreased to 5 wt% (n=6/group). * indicates p < 0.05 by ANOVA with Tukey’s correction for multiple comparisons. Adding a non-cytotoxic surfactant (0.25 wt% Tween 80) did not significantly change viability, however, it significantly increased the strength of the printed materials in 3 point bending (b) by enhancing binder solution printability into the powder. Therefore, 8.75 wt% phosphoric acid + 0.25 wt% Tween 80 was chosen as the baseline binder solution for subsequent experiments. # indicates p < 0.05 by 2-way ANOVA with Sidak’s correction for multiple comparisons. n=6–9/group. Bars represent means and error bars are standard deviation.
Figure 2. Optimization of powder particle size for 3D printing accuracy of murine-sized femoral scaffolds
a) 3D image renderings from micro-CT scans of 3 mm murine femoral scaffolds were used to measure the printing accuracy relative to the ideal computer-aided design (CAD) image, which was utilized to guide the printing process. b) Volumetric differences were quantified as the sum of deficient scaffold (where scaffold material should exist, but does not) and excess scaffold (where scaffold material exists, but should not) and were normalized to the total scaffold volume. Volumetric porosity (c) and pore size (d), estimated from the same micro-CT images, were primarily dictated by the lower limit of the powder particle size. * indicates significant differences (p < 0.05) from 30–50 µm powder by ANOVA with Dunnett’s correction for multiple comparisons. Absolute volumetric error was used for statistical comparison in (b). Bars represent means and error bars are standard deviation. n=4–5/group.
Figure 3. Scanning electron micrographs demonstrating a 3D printed murine femoral scaffold with micro-porosity and plate-like crystal formation
a) Murine-sized scaffold representing the geometry of the femoral mid-diaphysis for use in tissue engineering applications and preclinical bone regeneration studies. Scale bar is 250 µm. b,c) The 3D printing process produces scaffolds with intrinsic micro-porosity, primarily in the range of 20–50 µm, which is consistent with measurements from micro-CT images. Scale bars are 100 µm (b) and 10 µm (c). d) Plate-like crystal growth occurs on the surface of unreacted calcium phosphate particles, which increases the specific surface area for protein or drug adsorption. Scale bar is 1 µm.
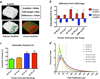
Figure 4. Viscosity adjustment of collagen solutions in phosphoric acid enables 3D inkjet printing of collagen-calcium phosphate composites
a) The viscosity of collagen in phosphoric acid solutions increases exponentially with collagen concentration at lower acidities (5 and 8.75 wt%), impeding inkjet printing. A brief heat treatment of collagen solutions at 37°C for 30 minutes dramatically reduces the viscosity (measured at 25°C) to ranges appropriate for inkjet printing. b) Interactions among collagen chains cause the exponential viscosity increase, as demonstrated by the shear thinning present with 0.3 wt% and 1 wt% collagen in 5 wt% phosphoric acid. With higher acid concentrations (≥12.5 wt%), or after heat treatment at 37°C for 30 minutes, the collagen is likely no longer in a fibrillar chain form. This denaturation results in Newtonian behavior and minimal changes in viscosity with collagen concentration. c) The viscosity of collagen solutions in 8.75 wt% phosphoric acid decreases dramatically after printing from the inkjets, which likely results from chain denaturation associated with the thermal printing process. d) The printed solutions were confirmed to maintain at least 94% collagen content based on the optical density (OD) at 280 nm, indicating that reductions in collagen concentration do not explain the reduced viscosity. e) Scanning electron micrograph demonstrating collagen (arrow), delivered via the inkjets, dispersed among the bound calcium phosphate crystals in a 3D printed scaffold. Scale bar is 5 µm.
Figure 5. Flexural strength and cell viability is significantly improved by collagen in the binder solution
a) Maximum flexural strength is significantly increased with increasing concentrations of collagen in the binder solution (8.75 wt% phosphoric acid + 0.25 wt% Tween 80). Points represent means and error bars are standard error of the mean. n=8–9/group. * indicates p < 0.05 by ANOVA with Tukey’s correction for multiple comparisons. Linear regression analysis confirms the slope is significantly different from zero with an r2 = 0.54. b) Group-averaged 3 point flexure stress-strain curves demonstrate the increasing strength with collagen in the binder solution (1 and 2 wt%), but a persistent brittleness of these materials. Coating the baseline 3D printed calcium phosphate scaffold (3DP-CPS) materials with a 0.5 wt% neutralized fibrillar collagen film significantly improves the flexural strength and toughness. c) Adding 1 wt% collagen into the binder solution significantly improves the relative viability (normalized to tissue culture plastic) of C3H/10T1/2 cells cultured on 3D printed discs relative to controls without collagen (3DP-CPS). # indicates p < 0.05 by ANCOVA comparing 3DP-CPS to 1 wt% collagen binder with culture time as the covariate. * indicates p < 0.05 compared to tissue culture plastic by ANOVA with Tukey’s correction for multiple comparisons. n=5/group. Bars represent means and error bars represent standard error of the mean. d) Pseudo-colored SEM image of a representative cell on a collagen-calcium phosphate composite that was 3D printed with 1 wt% collagen in the binder solution. Of note is that the cell is attached to and spreading out on the material, which is indicative of a healthy cell. Scale bar is 5 µm.
Figure 6. 3D printed calcium phosphate scaffolds are osteoconductive and enable bone ingrowth in a critically sized murine femoral defect
X-rays of the bone healing time course (a) and 3D micro-CT renderings at 9 weeks (b) demonstrate similar levels of new bone formation between allografts, 3D printed calcium phosphate scaffolds (3DP-CPS), and 3DP-CPSs with 1 wt% collagen supplementing the binder solution (Collagen Binder). 3DP-CPSs that were coated with a 0.5 wt% neutralized fibrillar collagen (Collagen Coated) tended to facilitate less new bone formation. c) The volume of newly formed bone that contained scaffold material (red; engraftment volume) was quantified through manual contouring coupled with automated edge detection in the 2D micro-CT slices.
Similar articles
- 3D fabrication and characterization of phosphoric acid scaffold with a HA/β-TCP weight ratio of 60:40 for bone tissue engineering applications.
Wang Y, Wang K, Li X, Wei Q, Chai W, Wang S, Che Y, Lu T, Zhang B. Wang Y, et al. PLoS One. 2017 Apr 13;12(4):e0174870. doi: 10.1371/journal.pone.0174870. eCollection 2017. PLoS One. 2017. PMID: 28406922 Free PMC article. - 3D-Printed Cell-Instructive Scaffolds Based on Chondrosia reniformis Collagen and Sr-Doped Calcium Phosphates for Bone Tissue Engineering.
Rocha MS, Marques CF, Pina S, Oliveira JM, Reis RL, Silva TH. Rocha MS, et al. ACS Biomater Sci Eng. 2025 Jun 9;11(6):3547-3559. doi: 10.1021/acsbiomaterials.4c01926. Epub 2025 May 6. ACS Biomater Sci Eng. 2025. PMID: 40326382 - Different post-processing conditions for 3D bioprinted α-tricalcium phosphate scaffolds.
Bertol LS, Schabbach R, Loureiro Dos Santos LA. Bertol LS, et al. J Mater Sci Mater Med. 2017 Sep 15;28(10):168. doi: 10.1007/s10856-017-5989-1. J Mater Sci Mater Med. 2017. PMID: 28916883 - 3D printing of inorganic-biopolymer composites for bone regeneration.
van der Heide D, Cidonio G, Stoddart MJ, D'Este M. van der Heide D, et al. Biofabrication. 2022 Sep 16;14(4). doi: 10.1088/1758-5090/ac8cb2. Biofabrication. 2022. PMID: 36007496 Review. - Three-dimensional (3D) printed scaffold and material selection for bone repair.
Zhang L, Yang G, Johnson BN, Jia X. Zhang L, et al. Acta Biomater. 2019 Jan 15;84:16-33. doi: 10.1016/j.actbio.2018.11.039. Epub 2018 Nov 24. Acta Biomater. 2019. PMID: 30481607 Review.
Cited by
- In Vitro Mineralization of Collagen.
Oosterlaken BM, Vena MP, de With G. Oosterlaken BM, et al. Adv Mater. 2021 Apr;33(16):e2004418. doi: 10.1002/adma.202004418. Epub 2021 Mar 12. Adv Mater. 2021. PMID: 33711177 Free PMC article. Review. - 3D bioprinting and craniofacial regeneration.
Dwivedi R, Mehrotra D. Dwivedi R, et al. J Oral Biol Craniofac Res. 2020 Oct-Dec;10(4):650-659. doi: 10.1016/j.jobcr.2020.08.011. Epub 2020 Aug 14. J Oral Biol Craniofac Res. 2020. PMID: 32983859 Free PMC article. Review. - AI-Driven Data Analysis of Quantifying Environmental Impact and Efficiency of Shape Memory Polymers.
Olawumi MA, Oladapo BI, Olugbade TO, Omigbodun FT, Olawade DB. Olawumi MA, et al. Biomimetics (Basel). 2024 Aug 14;9(8):490. doi: 10.3390/biomimetics9080490. Biomimetics (Basel). 2024. PMID: 39194469 Free PMC article. Review. - Main 3D Manufacturing Techniques for Customized Bone Substitutes. A Systematic Review.
Montero J, Becerro A, Pardal-Peláez B, Quispe-López N, Blanco JF, Gómez-Polo C. Montero J, et al. Materials (Basel). 2021 May 12;14(10):2524. doi: 10.3390/ma14102524. Materials (Basel). 2021. PMID: 34066290 Free PMC article. Review. - Phosphorene Is the New Graphene in Biomedical Applications.
Tatullo M, Genovese F, Aiello E, Amantea M, Makeeva I, Zavan B, Rengo S, Fortunato L. Tatullo M, et al. Materials (Basel). 2019 Jul 18;12(14):2301. doi: 10.3390/ma12142301. Materials (Basel). 2019. PMID: 31323844 Free PMC article. Review.
References
- De Long WG, Jr, Einhorn TA, Koval K, McKee M, Smith W, Sanders R, et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89(3):649–658. - PubMed
- Kurien T, Pearson RG, Scammell BE. Bone graft substitutes currently available in orthopaedic practice: the evidence for their use. Bone Joint J. 2013;95-B(5):583–597. - PubMed
- Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74(2):782–788. - PubMed
- Fierz FC, Beckmann F, Huser M, Irsen SH, Leukers B, Witte F, et al. The morphology of anisotropic 3D-printed hydroxyapatite scaffolds. Biomaterials. 2008;29(28):3799–3806. - PubMed
- Igawa K, Mochizuki M, Sugimori O, Shimizu K, Yamazawa K, Kawaguchi H, et al. Tailor-made tricalcium phosphate bone implant directly fabricated by a three-dimensional ink-jet printer. J Artif Organs. 2006;9(4):234–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AR061307/AR/NIAMS NIH HHS/United States
- P50 AR054041/AR/NIAMS NIH HHS/United States
- R01 AR056696/AR/NIAMS NIH HHS/United States
- P30AR061307/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources