Tick-borne flaviviruses antagonize both IRF-1 and type I IFN signaling to inhibit dendritic cell function - PubMed (original) (raw)
Tick-borne flaviviruses antagonize both IRF-1 and type I IFN signaling to inhibit dendritic cell function
Shelly J Robertson et al. J Immunol. 2014.
Abstract
Tick-borne encephalitis virus (TBEV), a member of the Flaviviridae family, is a leading cause of viral encephalitis in Europe and Asia. Dendritic cells (DCs), as early cellular targets of infection, provide an opportunity for flaviviruses to inhibit innate and adaptive immune responses. Flaviviruses modulate DC function, but the mechanisms underpinning this are not defined. We examined the maturation phenotype and function of murine bone marrow-derived DCs infected with Langat virus (LGTV), a naturally attenuated member of the TBEV serogroup. LGTV infection failed to induce DC maturation or a cytokine response. Treatment with LPS or LPS/IFN-γ, strong inducers of inflammatory cytokines, resulted in enhanced TNF-α and IL-6 production, but suppressed IL-12 production in infected DCs compared with uninfected "bystander" cells or mock-infected controls. LGTV-mediated antagonism of type I IFN (IFN-I) signaling contributed to inhibition of IL-12p40 mRNA expression at late time points after stimulation. However, early suppression was still observed in DCs lacking the IFN-I receptor (Ifnar(-/-)), suggesting that additional mechanisms of antagonism exist. The early IFN-independent inhibition of IL-12p40 was nearly abolished in DCs deficient in IFN regulatory factor-1 (IRF-1), a key transcription factor required for IL-12 production. LGTV infection did not affect Irf-1 mRNA expression, but rather diminished IRF-1 protein levels and nuclear localization. The effect on IRF-1 was also observed in DCs infected with the highly virulent Sofjin strain of TBEV. Thus, antagonism of IRF-1 is a novel mechanism that synergizes with the noted ability of flaviviruses to suppress IFN-α/β receptor-dependent signaling, resulting in the orchestrated evasion of host innate immunity.
Figures
Figure 1
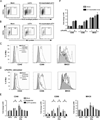
LGTV replication suppresses DC maturation. DCs were mock-infected, infected with LGTV strain TP21 or exposed to UV-inactivated LGTV. At 40 hpi, cells were stained for surface CD11c and intracellular viral protein and analyzed by flow cytometry. A and B, Representative dot plots of CD11c and viral envelope protein (A) or NS3 protein (B) shows the gating and percentage of LGTV-positive and LGTV-negative bystander populations. C, Expression of CD40, CD86, and MHCII on mock-infected (filled gray lines) and gated LGTV-positive cells (black line) are shown. DCs treated with LPS/IFNγ (gray dashed line) for 24 h served as a positive control for maturation. D, At 28 hpi, mock- and LGTV-infected DCs were cultured in the presence of various activation stimuli and changes in surface phenotype were assessed. Expression of CD40, CD86, and MHCII on LGTV-negative bystander (gray line) and LGTV-positive DCs (black line) following stimulation with LPS/IFNγ compared to mock-infected, untreated (filled gray lines) cells. E, Fold change in the geometric mean fluorescence intensity (MFI) of surface markers relative to isotype controls following no treatment or stimulation with LPS, LPS/IFNγ or poly I:C (PIC). Results are presented as the mean±SEM of three independent experiments. *P<0.05. F, The fold change in MFI of CD40, CD86 and MHCII following LPS/ IFNγ stimulation of DC exposed to UV-inactivated virus and mock-infected controls. Results from 1 of 2 independent experiments are presented as the mean±SD of triplicate samples.
Figure 2
Production of IL-12p40/p70, but not TNF-α, is suppressed in LGTV-infected DCs following TLR stimulation. DCs were mock-infected, or infected with LGTV for 28 h and then stimulated with LPS, LPS/IFNγ, or loxoribine for 8 h and analyzed by flow cytometry. Representative dot plots for viral protein and TNF-α (A) or IL-12p40/p70 (B) in CD11c-positive cells are shown. The percentage of cells expressing TNF-α (C) or IL-12p40/p70 (D) for each population as indicated is shown. *P<0.05. E, The percentage of DCs, mock-infected or exposed to UV-inactivated virus, that express IL-12p40/p70 following stimulation with LPS, or LPS/IFNγ. Results are presented as the mean±SD of three independent experiments.
Figure 3
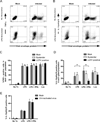
LGTV infection of DCs inhibits IL-12 production and T cell proliferative capacity. DCs were mock-infected or infected with LGTV for 28 h and then treated with medium alone, or medium containing LPS/IFNγ or loxoribine. A, Culture supernatants were harvested at 0, 3, 8, and 24 h following stimulation and levels of IL-12p40, IL-12p70, TNF-α, IL-6, and IL-10 were determined by Bio-plex immunoassay. Data are compiled from three independent experiments and expressed as the mean±SD. *P<0.05. B, DC infection and LPS/IFNγ stimulation was performed in the presence of anti-IL10R or RtIgG control antibodies. Cells were stained for intracellular IL-12p40 at 8 h post-stimulation and analyzed by flow cytometry (upper graph) or supernatants were collected at 24 h post-stimulation and secreted cytokines were measured by Bioplex (lower graph). The graphs represent the mean ± SD of cells expressing IL-12p40 in each population or of IL-12p40 production. Data is representative of 2 independent experiments performed in triplicate. *P<0.05. C, At 24 h post-stimulation, DCs were loaded with Ova323 peptide and cocultured with CFSE-labeled, Ova-specific CD4+ T cells at a ratio of 1:5 (DCs to T cells). After 4 days, cells were harvested and analyzed by flow cytometry. The dot plots (CFSE versus CD3) show the loss of CFSE fluorescence in T cells (identified by CD3 positive staining) cocultured with untreated or LPS/IFNγ-stimulated DCs. Representative dot blots show the percentage of proliferating cells, as indicated by the boxed gate, among total gated live T cells. The graph represents the mean ± SD of proliferating cells from three independent experiments. *P<0.05.
Figure 4
LGTV impairment of DC function is partially independent of IFN-I signaling. DCs derived from _Ifnar_−/− mice were mock-infected or infected with LGTV for 28 h and then stimulated with LPS, LPS/IFNγ, or loxoribine. A, Percentages of mock-infected, LGTV-positive, and LGTV-negative bystander DCs expressing IL-12p40/p70 as determined by flow cytometry. Data are presented as the mean ± SD of three independent experiments. *P<0.05. B, At 24 h post-stimulation, _Ifnar_−/− DCs were loaded with Ova323 peptide and cocultured with CFSE-labeled, Ova-specific CD4+ T cells. After 4 days, cells were harvested and analyzed by flow cytometry. The graph represents the mean ± SD of proliferating cells from three independent experiments. *P<0.05.
Figure 5
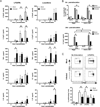
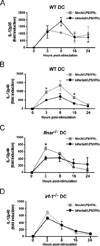
LGTV suppresses IL-12p40 mRNA expression by a mechanism dependent on IFN-I signaling and IRF-1. DCs derived from WT, _Ifnar_−/− or _Irf-1_−/− mice were mock-infected or infected with LGTV for 28 h and then stimulated with LPS/IFNγ. IL-12p35 and IL-12p40 mRNA expression was determined by quantitative RT-PCR and the fold induction was calculated relative to mock-infected, untreated controls. The graphs show the kinetics of mRNA expression for IL-12p35 in WT DCs(A) or IL-12p40 in WT DCs (B), _Ifnar_−/− DCs (C) and _Irf-1_−/− DCs (D). Data represent mean fold induction ± SEM of at least 3 independent experiments. *P<0.05 compared to mock-infected, treated at the same time point.
Figure 6
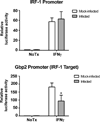
LGTV attenuates IRF-1 transcriptional activity. Huh7 cells were transfected with l IRF-1 or Gbp-2 luciferase reporter plasmids 24 h prior to infection with LGTV (MOI 3). At 24 hpi, cells were treated with or without IFNγ for 6 h and luciferase enzyme luminescent activity was measured. Luciferase activity was normalized to the constitutive luciferase activity of pTK-Renilla and expressed as fold change over the activity of mock-infected, unstimulated control. Data represent the mean ± SD of the fold change from three independent experiments. *P<0.05.
Figure 7
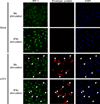
LGTV inhibits IRF-1 nuclear localization. At 28 h post-infection, mock- and LGTV-infected WT DCs were treated with LPS/IFNγ for 3 h and analyzed by immunofluorescence. Cells were stained for IRF-1 (green) and viral envelope protein (red). Nuclei were counterstained with DAPI (blue). Examples of cells that are positive for viral envelope protein and show diminished nuclear IRF-1 staining are indicated by the arrowheads in all viral envelope protein, IRF-1 and DAPI panels. Images are representative of three independent experiments.
Figure 8
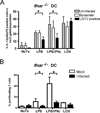
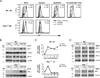
Flavivirus infection of DCs diminishes IRF-1 protein levels without affecting Irf-1 mRNA expression. WT or _Ifnar_−/− DCs were mock-infected, infected with LGTV or exposed to UV-inactivated virus for 28 h. Cells were treated with medium alone, or medium containing LPS/IFNγ. A, Histograms for intracellular IRF-1 expression are shown for mock-infected DC cultures, gated infected cells or bystander cells from infected cultures and DCs exposed to UV-inactivated virus as indicated. Most DCs (70–90%) were infected in _Ifnar_−/− cultures and therefore IRF-1 expression in bystander cells was not determined. MFI values of IRF-1 expression in untreated and stimulated samples are shown. B, Total protein lysates were assayed for IRF-1 and IRF-8 by immunoblot. Duplicate blots were probed for β-actin as a loading control. C, RNA was isolated at various times post-stimulation and Irf-1 mRNA expression was determined by quantitative RT-PCR. The data shown are representative of three independent experiments. D, WT and _Ifnar_−/− DCs were infected with TBEV (Sofjin strain) for 28h and left untreated, or stimulated with LPS or LPS/IFNγ for 3 h. IRF-1 and IRF-8 were detected by immunoblot. Blots were stripped and reprobed for β-actin. A representative immunoblot of three independent experiments for both LGTV and TBEV is shown.
Similar articles
- Type I interferon protects mice from fatal neurotropic infection with Langat virus by systemic and local antiviral responses.
Weber E, Finsterbusch K, Lindquist R, Nair S, Lienenklaus S, Gekara NO, Janik D, Weiss S, Kalinke U, Överby AK, Kröger A. Weber E, et al. J Virol. 2014 Nov;88(21):12202-12. doi: 10.1128/JVI.01215-14. Epub 2014 Aug 13. J Virol. 2014. PMID: 25122777 Free PMC article. - Inhibition of interferon-stimulated JAK-STAT signaling by a tick-borne flavivirus and identification of NS5 as an interferon antagonist.
Best SM, Morris KL, Shannon JG, Robertson SJ, Mitzel DN, Park GS, Boer E, Wolfinbarger JB, Bloom ME. Best SM, et al. J Virol. 2005 Oct;79(20):12828-39. doi: 10.1128/JVI.79.20.12828-12839.2005. J Virol. 2005. PMID: 16188985 Free PMC article. - Type I interferon regulates respiratory virus infected dendritic cell maturation and cytokine production.
Rudd BD, Luker GD, Luker KE, Peebles RS, Lukacs NW. Rudd BD, et al. Viral Immunol. 2007 Dec;20(4):531-40. doi: 10.1089/vim.2007.0057. Viral Immunol. 2007. PMID: 18158727 - IRF family proteins and type I interferon induction in dendritic cells.
Tailor P, Tamura T, Ozato K. Tailor P, et al. Cell Res. 2006 Feb;16(2):134-40. doi: 10.1038/sj.cr.7310018. Cell Res. 2006. PMID: 16474425 Review. - Sources of Type I Interferons in Infectious Immunity: Plasmacytoid Dendritic Cells Not Always in the Driver's Seat.
Ali S, Mann-Nüttel R, Schulze A, Richter L, Alferink J, Scheu S. Ali S, et al. Front Immunol. 2019 Apr 12;10:778. doi: 10.3389/fimmu.2019.00778. eCollection 2019. Front Immunol. 2019. PMID: 31031767 Free PMC article. Review.
Cited by
- In Vitro Characterization of the Innate Immune Pathways Engaged by Live and Inactivated Tick-Borne Encephalitis Virus.
Signorazzi A, Pennings JLA, Etna MP, Noya M, Coccia EM, Huckriede A. Signorazzi A, et al. Vaccines (Basel). 2021 Jun 17;9(6):664. doi: 10.3390/vaccines9060664. Vaccines (Basel). 2021. PMID: 34204532 Free PMC article. - The Role of IFITM Proteins in Tick-Borne Encephalitis Virus Infection.
Chmielewska AM, Gómez-Herranz M, Gach P, Nekulova M, Bagnucka MA, Lipińska AD, Rychłowski M, Hoffmann W, Król E, Vojtesek B, Sloan RD, Bieńkowska-Szewczyk K, Hupp T, Ball K. Chmielewska AM, et al. J Virol. 2022 Jan 12;96(1):e0113021. doi: 10.1128/JVI.01130-21. Epub 2021 Oct 6. J Virol. 2022. PMID: 34613785 Free PMC article. - Difference in Intraspecies Transmissibility of Severe Fever with Thrombocytopenia Syndrome Virus Depending on Abrogating Type 1 Interferon Signaling in Mice.
Oh B, Park SC, Yang MS, Yang D, Ham G, Tark D, You MJ, Oh SI, Kim B. Oh B, et al. Viruses. 2024 Mar 5;16(3):401. doi: 10.3390/v16030401. Viruses. 2024. PMID: 38543766 Free PMC article. - Interferon signaling in Peromyscus leucopus confers a potent and specific restriction to vector-borne flaviviruses.
Izuogu AO, McNally KL, Harris SE, Youseff BH, Presloid JB, Burlak C, Munshi-South J, Best SM, Taylor RT. Izuogu AO, et al. PLoS One. 2017 Jun 26;12(6):e0179781. doi: 10.1371/journal.pone.0179781. eCollection 2017. PLoS One. 2017. PMID: 28650973 Free PMC article. - Flaviviruses: Innate Immunity, Inflammasome Activation, Inflammatory Cell Death, and Cytokines.
Pan Y, Cai W, Cheng A, Wang M, Yin Z, Jia R. Pan Y, et al. Front Immunol. 2022 Jan 28;13:829433. doi: 10.3389/fimmu.2022.829433. eCollection 2022. Front Immunol. 2022. PMID: 35154151 Free PMC article. Review.
References
- Gritsun TS, Lashkevich VA, Gould EA. Tick-borne encephalitis. Antiviral Res. 2003;57:129–146. - PubMed
- Lindquist L, Vapalahti O. Tick-borne encephalitis. Lancet. 2008;371:1861–1871. - PubMed
- Mansfield KL, Johnson N, Phipps LP, Stephenson JR, Fooks AR, Solomon T. Tick-borne encephalitis virus - a review of an emerging zoonosis. J. Gen. Virol. 2009;90:1781–1794. - PubMed
- Dobler G. Zoonotic tick-borne flaviviruses. Vet. Microbiol. 2010;140:221–228. - PubMed
- Kawai T, Akira S. Innate immune recognition of viral infection. Nat. Immunol. 2006;7:131–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases