The Hippo-YAP signaling pathway and contact inhibition of growth - PubMed (original) (raw)
The Hippo-YAP signaling pathway and contact inhibition of growth
Barry M Gumbiner et al. J Cell Sci. 2014.
Abstract
The Hippo-YAP pathway mediates the control of cell proliferation by contact inhibition as well as other attributes of the physical state of cells in tissues. Several mechanisms sense the spatial and physical organization of cells, and function through distinct upstream modules to stimulate Hippo-YAP signaling: adherens junction or cadherin-catenin complexes, epithelial polarity and tight junction complexes, the FAT-Dachsous morphogen pathway, as well as cell shape, actomyosin or mechanotransduction. Soluble extracellular factors also regulate Hippo pathway signaling, often inhibiting its activity. Indeed, the Hippo pathway mediates a reciprocal relationship between contact inhibition and mitogenic signaling. As a result, cells at the edges of a colony, a wound in a tissue or a tumor are more sensitive to ambient levels of growth factors and more likely to proliferate, migrate or differentiate through a YAP and/or TAZ-dependent process. Thus, the Hippo-YAP pathway senses and responds to the physical organization of cells in tissues and coordinates these physical cues with classic growth-factor-mediated signaling pathways. This Commentary is focused on the biological significance of Hippo-YAP signaling and how upstream regulatory modules of the pathway interact to produce biological outcomes.
Keywords: Cadherin; Hippo; Mechanotransduction; Mitogenesis; Polarity; YAP.
Figures
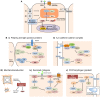
Fig. 1.
Control of the core Hippo signaling pathway through interacting upstream modules. (A) Overview of the interactions of various modules with the core pathway. The Hippo pathway consists of a core kinase cascade in which the transcriptional co-activators YAP/TAZ are phosphorylated and inactivated by either their exclusion from the nucleus or their enhanced degradation. The nuclear activity of YAP/TAZ promotes cell growth. (B) Upstream modules. (Panels i, ii) Two upstream cell surface regulators, epithelial polarity or tight junction (TJ) complexes (i) and adherens junction (AJ) or cadherin–catenin complexes may function together to sense the integrity of the epithelial layer. (Panel iii) Cell shape and mechanotransduction can regulate the activity of YAP/TAZ independently of Lats kinase, but Lats-dependent regulation of YAP/TAZ through the actin cytoskeleton has also been observed. (Panel iv) Extracellular soluble growth factors act reciprocally – with contact inhibition – through the Hippo pathway to integrate mitogenesis with growth inhibitory mechanisms. (Panel v) The atypical cadherins FAT and Dachsous set up a morphogen gradient to control the spatial patterning of both cell proliferation (through Hippo pathway signaling) and PCP. β-cat, β-catenin; α-cat, α-catenin, AP, apical polarity complexes; Dco, Discs overgrown; E-cad, E-cadherin; ECM, extracellular matrix; ex, Expanded; GPCRs, G-protein-coupled receptors; RTK, receptor tyrosine kinase; PCP, planar cell polarity.
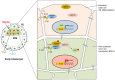
Fig. 2.
Hippo pathway-mediated regulation of cell lineage and pluripotency by adhesion and epithelial polarization in the early mouse embryo. Compaction of the mouse embryo at the pre-implantation stage leads to the development of two different cell lineages, trophectoderm (TE) and inner cell mass (ICM) (shown on the left). The TE is a polarized epithelium with E-cadherin (E-cad)-mediated adherens junctions (AJs), tight junctions (TJs) and apical–basolateral polarity (illustrated on the right); it differentiates into an extra-embryonic tissue, the placenta. The ICM contains adhesive cells that are non-polarized and form pluripotent embryonic stem cells (ESCs), which give rise to the embryo proper. Recruitment of angiomotin (AMOT) to the E-cad–catenin complexes of inner cells stimulates Hippo pathway signaling and restricts the transcriptional activity of YAP through its exclusion from the nucleus, thereby allowing expression of the pluripotency gene Nanog. AMOT becomes restricted to the apical domain of the outer cells by the action of polarity protein complexes during epithelialization, which leads to a loss of Hippo pathway signaling, nuclear accumulation of YAP and transcriptional activation of TE-specific transcription factor Cdx2. β-cat, β-catenin; α-cat, α-catenin.
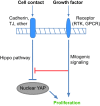
Fig. 3.
The Hippo pathway mediates the reciprocal regulation of cell proliferation by contact inhibition and mitogenic signaling. Cell-cell contact inhibits cell proliferation through stimulation of the Hippo pathway. Mitogenic growth factors stimulate proliferation by well-known signaling mechanisms, but also counteract the growth inhibitory effects of the Hippo signaling pathway. Thus, the Hippo pathway can coordinate classic mitogenic signaling with the physical state of the cells in a tissue. TJ, tight junction; RTK, receptor tyrosine kinase; GPCR, G-protein-coupled receptor.
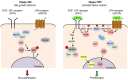
Fig. 4.
Two mechanisms for growth-factor-mediated regulation of the Hippo pathway: activation of Ras-MAPK signaling and activation of PI3K-PDK1 signaling. (Left panel, Hippo pathway on) In confluent cells in the absence of growth factors, PDK1 forms a complex with Hippo pathway components (Lats, Mst and Sav1) and the Hippo pathway is active. Mst phosphorylates Mob and Lats, which then phosphorylate YAP. YAP is then excluded from the nucleus and cell growth is arrested. (Right panel, Hippo pathway off) Activation of the Ras-MAPK pathway by EGF signaling phosphorylates Ajuba, which binds to and inhibits the activity of the Sav�–Wts complex, leading to dephosphorylation of Yorkie, its accumulation in the nucleus and increased cell proliferation. Growth factors (EGF, LPA or serum) can also activate PI3K and recruit PDK1 to the membrane, resulting in the dissociation of the PDK1–Hippo complex. As a result, the regulation of Lats by Mst is prevented, which eventually leads to nuclear accumulation of YAP and cell proliferation. Figure was modified with permission (Fan et al., 2013). PIP2, PtdIns(4,5)_P_2; PIP3, PtdIns(3,4,5)_P_3.
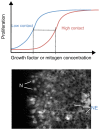
Fig. 5.
Role of contact inhibition and Hippo-YAP signaling in the spatial control of mitogenic signaling by growth factors. (Top) The graph shows the dose-response curves for growth factor-stimulated proliferation. A high degree of cell-cell contact shifts the curve on the _x_-axis, such that a higher concentration of growth factors is required to elicit the same response. This graph is an interpretation of the findings of Kim et al., 2009. (Bottom) Illustration how cell-cell contact regulates transcription factor activity through the Hippo pathway. At high cell density in the middle of the cell monolayer, the Hippo pathway is active, leading to nuclear exclusion (NE) of YAP and/or TEAD (TEAD is labeled by immunofluorescence staining in this example). At the edge of the culture where cells have lost contact inhibition, YAP and/or TEAD (as shown here) accumulate in the nuclei (N) and stimulate proliferation. Thus, proliferation is spatially regulated despite uniform levels of growth factors acting on these cells.
Similar articles
- E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components.
Kim NG, Koh E, Chen X, Gumbiner BM. Kim NG, et al. Proc Natl Acad Sci U S A. 2011 Jul 19;108(29):11930-5. doi: 10.1073/pnas.1103345108. Epub 2011 Jul 5. Proc Natl Acad Sci U S A. 2011. PMID: 21730131 Free PMC article. - Regulation of YAP/TAZ Activity by Mechanical Cues: An Experimental Overview.
Dupont S. Dupont S. Methods Mol Biol. 2019;1893:183-202. doi: 10.1007/978-1-4939-8910-2_15. Methods Mol Biol. 2019. PMID: 30565135 Review. - Reciprocal regulation of YAP/TAZ by the Hippo pathway and the Small GTPase pathway.
Jang JW, Kim MK, Bae SC. Jang JW, et al. Small GTPases. 2020 Jul;11(4):280-288. doi: 10.1080/21541248.2018.1435986. Epub 2018 Apr 20. Small GTPases. 2020. PMID: 29457552 Free PMC article. Review. - Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1.
Fan R, Kim NG, Gumbiner BM. Fan R, et al. Proc Natl Acad Sci U S A. 2013 Feb 12;110(7):2569-74. doi: 10.1073/pnas.1216462110. Epub 2013 Jan 28. Proc Natl Acad Sci U S A. 2013. PMID: 23359693 Free PMC article. - Differential regulation of the Hippo pathway by adherens junctions and apical-basal cell polarity modules.
Yang CC, Graves HK, Moya IM, Tao C, Hamaratoglu F, Gladden AB, Halder G. Yang CC, et al. Proc Natl Acad Sci U S A. 2015 Feb 10;112(6):1785-90. doi: 10.1073/pnas.1420850112. Epub 2015 Jan 26. Proc Natl Acad Sci U S A. 2015. PMID: 25624491 Free PMC article.
Cited by
- SHANK2 is a frequently amplified oncogene with evolutionarily conserved roles in regulating Hippo signaling.
Xu L, Li P, Hao X, Lu Y, Liu M, Song W, Shan L, Yu J, Ding H, Chen S, Yang A, Zeng YA, Zhang L, Jiang H. Xu L, et al. Protein Cell. 2021 Mar;12(3):174-193. doi: 10.1007/s13238-020-00742-6. Epub 2020 Jul 13. Protein Cell. 2021. PMID: 32661924 Free PMC article. - Deregulation of the Hippo pathway in mouse mammary stem cells promotes mammary tumorigenesis.
Li H, Gumbiner BM. Li H, et al. Mamm Genome. 2016 Dec;27(11-12):556-564. doi: 10.1007/s00335-016-9662-7. Epub 2016 Sep 6. Mamm Genome. 2016. PMID: 27601049 Free PMC article. - Upstream regulation of the Hippo-Yap pathway in cardiomyocyte regeneration.
Flinn MA, Link BA, O'Meara CC. Flinn MA, et al. Semin Cell Dev Biol. 2020 Apr;100:11-19. doi: 10.1016/j.semcdb.2019.09.004. Epub 2019 Oct 9. Semin Cell Dev Biol. 2020. PMID: 31606277 Free PMC article. Review. - YAP and the RhoC regulator ARHGAP18, are required to mediate flow-dependent endothelial cell alignment.
Coleman PR, Lay AJ, Ting KK, Zhao Y, Li J, Jarrah S, Vadas MA, Gamble JR. Coleman PR, et al. Cell Commun Signal. 2020 Feb 3;18(1):18. doi: 10.1186/s12964-020-0511-7. Cell Commun Signal. 2020. PMID: 32013974 Free PMC article. - Heterozygous IDH1R132H/WT created by "single base editing" inhibits human astroglial cell growth by downregulating YAP.
Wei S, Wang J, Oyinlade O, Ma D, Wang S, Kratz L, Lal B, Xu Q, Liu S, Shah SR, Zhang H, Li Y, Quiñones-Hinojosa A, Zhu H, Huang ZY, Cheng L, Qian J, Xia S. Wei S, et al. Oncogene. 2018 Sep;37(38):5160-5174. doi: 10.1038/s41388-018-0334-9. Epub 2018 May 30. Oncogene. 2018. PMID: 29849122 Free PMC article.
References
- Adler J. J., Johnson D. E., Heller B. L., Bringman L. R., Ranahan W. P., Conwell M. D., Sun Y., Hudmon A., Wells C. D. (2013). Serum deprivation inhibits the transcriptional co-activator YAP and cell growth via phosphorylation of the 130-kDa isoform of Angiomotin by the LATS1/2 protein kinases. Proc. Natl. Acad. Sci. USA 110, 17368–17373 10.1073/pnas.1308236110 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials