Synergism from the combination of ulinastatin and curcumin offers greater inhibition against colorectal cancer liver metastases via modulating matrix metalloproteinase-9 and E-cadherin expression - PubMed (original) (raw)
Synergism from the combination of ulinastatin and curcumin offers greater inhibition against colorectal cancer liver metastases via modulating matrix metalloproteinase-9 and E-cadherin expression
Fei Shen et al. Onco Targets Ther. 2014.
Erratum in
Abstract
Liver metastasis is a major cause of mortality in colorectal cancer (CRC). The current study was to investigate the ability of ulinastatin (UTI) and curcumin (CUR) to inhibit CRC liver metastases via modulating matrix metalloproteinase-9 (MMP-9) and E-cadherin expression. Human CRC HCT-116 cells were treated with compounds individually and in combination in order to understand the effect on cell migration and invasion. The HCT-116 cell line was established to stably express luciferase and green fluorescent protein (GFP) by lentiviral transduction (HCT-116-Luc-GFP). We identified an anti-metastasis effect of UTI and CUR on a CRC liver metastasis mouse model. Tumor development and therapeutic responses were dynamically tracked by bioluminescence imaging. Expression of MMP-9 and E-cadherin in metastatic tumors was detected by immunohistochemical assay. Results of wound healing and cell invasion assays suggest that treatment with UTI, CUR, and UTI plus CUR, respectively, significantly inhibit HCT-116 cell migration and invasion. Furthermore, results of CRC hepatic metastasis on a nude mouse model showed that treatment with UTI, CUR alone, and a combination notably inhibited hepatic metastases from CRC and prolonged survival of tumor-bearing mice, especially in the UTI plus CUR group. These results suggest that the combination of UTI and CUR together may offer greater inhibition against metastasis of CRC.
Keywords: bioluminescence imaging; hepatic metastasis; therapy.
Figures
Figure 1
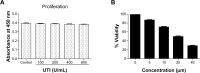
Effect of ulinastatin (UTI) or curcumin (CUR) on cell viability and proliferation of HCT-116. (A) Cytotoxicity of UTI against HCT-116 cells in vitro. Tumor cells (5.0 × 103) in each well of a 96-well culture plate were incubated for 24 hours at 37°C with or without various concentrations of UTI. CCK-8 was added to each well and, after incubation for 2 hours, the absorbance was measured at 450 nm. (B) HCT-116 cells were treated with different concentrations of CUR for 24 hours, and cell viability was measured using the CCK-8 method. Concentrations of CUR resulting in 50% growth inhibition were indicated as individual IC50 (50% cell growth inhibitory concentrations) values.
Figure 2
Effect of ulinastatin (UTI) and curcumin (CUR) on cell migration and invasion. (A) Migration of HCT-116 was assayed by wound healing assay. Cells were cultured to nearly confluent cell monolayer. A scratch wound was created on the cell surface using a micropipette tip. The monolayer was washed with phosphate buffered saline, and then UTI (800 U) or CUR (10 μM) was added or not. The cultures were incubated at 37°C for 0 hours, 24 hours, and 48 hours, respectively, and pictures were taken using light microscopy (×100). (B) The width of the wound was measured and the wound closure rate was calculated. (C) Transwell in vitro invasion assay detects the effect of UTI and CUR on the invasive ability of colon cancer cells. (a) cells treated with PBS; (b) cells treated with UTI; (c) cells treated with CUR; (d) cells treated with UTI and CUR. (D) The invaded cell numbers were measured and compared. Note: *P<0.05 versus control.
Figure 2
Effect of ulinastatin (UTI) and curcumin (CUR) on cell migration and invasion. (A) Migration of HCT-116 was assayed by wound healing assay. Cells were cultured to nearly confluent cell monolayer. A scratch wound was created on the cell surface using a micropipette tip. The monolayer was washed with phosphate buffered saline, and then UTI (800 U) or CUR (10 μM) was added or not. The cultures were incubated at 37°C for 0 hours, 24 hours, and 48 hours, respectively, and pictures were taken using light microscopy (×100). (B) The width of the wound was measured and the wound closure rate was calculated. (C) Transwell in vitro invasion assay detects the effect of UTI and CUR on the invasive ability of colon cancer cells. (a) cells treated with PBS; (b) cells treated with UTI; (c) cells treated with CUR; (d) cells treated with UTI and CUR. (D) The invaded cell numbers were measured and compared. Note: *P<0.05 versus control.
Figure 3
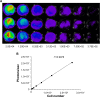
Measurement of bioluminescence in HCT-116-Luc-GFP cells. HCT-116-Luc-GFP cells were counted with a hemocytometer and plated in a 96-well plate at various concentrations. The plate was imaged to verify that the cells bioluminesced in a concentration-dependent manner. (A) Quantitation of bioluminescence (photons/sec) was graphed. (B) Linear regression analysis showed a good correlation between cell number and mean bioluminescence imaging or fluorescent intensity (_r_=0.9978).
Figure 4
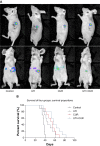
Ulinastatin (UTI) and curcumin (CUR) inhibits liver metastasis and prolongs survival. (A) Luciferase-expressing HCT-116 cells were injected into the spleens of BLAB/c mice. The control group (n=7) received vehicle, the UTI group (n=7) was injected with UTI at 8,000 U/mouse once daily, the CUR group (n=7) was administered orally CUR alone (1 g/kg) once daily, and the UTI plus CUR group (n=7) was treated with a combination of UTI (8,000 U/mouse) and CUR (1 g/kg). Therapy was continued for 4 weeks. Bioluminescence imaging was used to monitor liver metastasis of HCT-116-Luc-GFP cells in vivo 1 day and 28 days after splenic injection. (B) Survival of mice treated with/without UTI and/or CUR was assayed after injecting HCT-116-Luc-GFP cells.
Figure 5
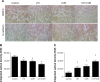
Expression of MMP-9 and E-cadherin in metastatic tumors was detected by immunohistochemical assay (original magnification 400×). (A) Expression of MMP-9 and E-cadherin in four groups. Differences in MMP-9 (B) and E-cadherin (C) protein expression are shown. Note: *P<0.05. Abbreviations: CUR, curcumin; MMP-9, modulating matrix metalloproteinase-9; UTI, ulinastatin.
Similar articles
- Curcumin enhances drug sensitivity of gemcitabine-resistant lung cancer cells and inhibits metastasis.
Dong Z, Feng Q, Zhang H, Liu Q, Gong J. Dong Z, et al. Pharmazie. 2021 Nov 1;76(11):538-543. doi: 10.1691/ph.2021.0927. Pharmazie. 2021. PMID: 34782038 - Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression.
Ji Q, Liu X, Han Z, Zhou L, Sui H, Yan L, Jiang H, Ren J, Cai J, Li Q. Ji Q, et al. BMC Cancer. 2015 Mar 5;15:97. doi: 10.1186/s12885-015-1119-y. BMC Cancer. 2015. PMID: 25884904 Free PMC article. - Curcumin suppresses colorectal cancer by induction of ferroptosis via regulation of p53 and solute carrier family 7 member 11/glutathione/glutathione peroxidase 4 signaling axis.
Ming T, Lei J, Peng Y, Wang M, Liang Y, Tang S, Tao Q, Wang M, Tang X, He Z, Liu X, Xu H. Ming T, et al. Phytother Res. 2024 Aug;38(8):3954-3972. doi: 10.1002/ptr.8258. Epub 2024 Jun 4. Phytother Res. 2024. PMID: 38837315 - Pea3 expression promotes the invasive and metastatic potential of colorectal carcinoma.
Mesci A, Taeb S, Huang X, Jairath R, Sivaloganathan D, Liu SK. Mesci A, et al. World J Gastroenterol. 2014 Dec 14;20(46):17376-87. doi: 10.3748/wjg.v20.i46.17376. World J Gastroenterol. 2014. PMID: 25516649 Free PMC article.
Cited by
- Effects of Ulinastatin on Proliferation and Apoptosis of Breast Cancer Cells by Inhibiting the ERK Signaling Pathway.
Xing Z, Wang X, Liu J, Liu G, Zhang M, Feng K, Wang X. Xing Z, et al. Biomed Res Int. 2021 Jul 30;2021:9999268. doi: 10.1155/2021/9999268. eCollection 2021. Biomed Res Int. 2021. PMID: 34373837 Free PMC article. Retracted. - The adaptation of colorectal cancer cells when forming metastases in the liver: expression of associated genes and pathways in a mouse model.
Bocuk D, Wolff A, Krause P, Salinas G, Bleckmann A, Hackl C, Beissbarth T, Koenig S. Bocuk D, et al. BMC Cancer. 2017 May 19;17(1):342. doi: 10.1186/s12885-017-3342-1. BMC Cancer. 2017. PMID: 28525976 Free PMC article. - Targeting E-cadherin expression with small molecules for digestive cancer treatment.
Song Y, Ye M, Zhou J, Wang Z, Zhu X. Song Y, et al. Am J Transl Res. 2019 Jul 15;11(7):3932-3944. eCollection 2019. Am J Transl Res. 2019. PMID: 31396310 Free PMC article. Review. - Identification of Hub Genes Related to Liver Metastasis of Colorectal Cancer by Integrative Analysis.
Liu S, Zhang Y, Zhang S, Qiu L, Zhang B, Han J. Liu S, et al. Front Oncol. 2021 Aug 19;11:714866. doi: 10.3389/fonc.2021.714866. eCollection 2021. Front Oncol. 2021. PMID: 34490113 Free PMC article. - The multifaceted role of curcumin in cancer prevention and treatment.
Shanmugam MK, Rane G, Kanchi MM, Arfuso F, Chinnathambi A, Zayed ME, Alharbi SA, Tan BK, Kumar AP, Sethi G. Shanmugam MK, et al. Molecules. 2015 Feb 5;20(2):2728-69. doi: 10.3390/molecules20022728. Molecules. 2015. PMID: 25665066 Free PMC article. Review.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
- Carneiro P, Figueiredo J, Bordeira-Carrico R, et al. Therapeutic targets associated to E-cadherin dysfunction in gastric cancer. Expert Opin Ther Targets. 2013;17:1187–1201. - PubMed
- Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–3654. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous