Dom34 rescues ribosomes in 3' untranslated regions - PubMed (original) (raw)
Dom34 rescues ribosomes in 3' untranslated regions
Nicholas R Guydosh et al. Cell. 2014.
Abstract
Ribosomes that stall before completing peptide synthesis must be recycled and returned to the cytoplasmic pool. The protein Dom34 and cofactors Hbs1 and Rli1 can dissociate stalled ribosomes in vitro, but the identity of targets in the cell is unknown. Here, we extend ribosome profiling methodology to reveal a high-resolution molecular characterization of Dom34 function in vivo. Dom34 removes stalled ribosomes from truncated mRNAs, but, in contrast, does not generally dissociate ribosomes on coding sequences known to trigger stalling, such as polyproline. We also show that Dom34 targets arrested ribosomes near the ends of 3' UTRs. These ribosomes appear to gain access to the 3' UTR via a mechanism that does not require decoding of the mRNA. These results suggest that ribosomes frequently enter downstream noncoding regions and that Dom34 carries out the important task of rescuing them.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
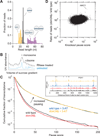
Figure 1. Analysis of Ribosome Footprint Size and Pausing
(A) Size distributions of untrimmed ribosome footprints that mapped without mismatches to the genome or splice junctions. Short monosome-protected fragments (15–24 nt, orange), long monosome-protected fragments (25–34 nt, black), and disome-protected fragments (40–80 nt, purple). Each distribution came from a separate sample and thus sums to 1.0. Black and purple distributions obtained from wild-type cells and orange from _ski2_Δ cells. (B) Sucrose gradient profile for _dom34_Δ yeast showing both RNase-treated and untreated control samples. Monosome and disome peaks are indicated. (C) Effect of DOM34 knockout and 3-AT on ribosome pausing. Cumulative histogram of pause score values (smoothed reads at a position / median reads in gene) computed for all positions in the transcriptome with adequate read density. (Inset) Ribosome footprint reads that map to an example gene (PGK1). Histidine codons are marked with gray arrowheads. (D) Enrichment ratio of reads mapped at single nucleotide positions in the _dom34_Δ strain compared to the wild-type strain shown as a function of knockout pause score (smoothed reads at a position / average reads in gene) for all positions in the transcriptome with adequate read density. Sites where pauses are amplified by knockout of DOM34, such as on HAC1, are located in the upper right quadrant. See also Figures S1, S2, and S6.
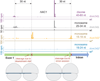
Figure 2. Dom34 Targets Ribosomes on a Truncated mRNA
Mapped footprints of varied sizes from wild-type (darker shades) and _dom34_Δ (lighter shades) strains on the gene HAC1. Data were collected from the disome and monosome fractions of a sucrose gradient. Note that scales vary according to sample and reads are plotted at approximate position of the ribosome A site. Ribosome drawings below the plots illustrate how the first set of ribosomes stall at the end of the exon and the second set of ribosomes stall at an upstream cleavage site, presumably caused by the stalled ribosomes downstream. Data for short (<25 nt) reads were taken in a _ski2_Δ background (indicated by *) to inhibit NSD. See also Figures S3 and S4.
Figure 3. Dom34 Targets Arrested Ribosomes in the 3´ UTR
(A) Enrichment ratio of reads on genes is plotted for 3´ UTRs as a function of ORFs between wild-type and _dom34_Δ strains. Vertical asymmetry of distribution indicates read enrichment in the _dom34_Δ 3´ UTRs. Genes were included with >10 rpkm in the ORF and >0.17 rpm (5–6 raw reads) in the 3´ UTR in both strains. (B) Examples of mapped footprints on genes (SGE1 and CPR5) showing enrichment near the end of the 3´ UTR in the _dom34_Δ strain. Reads that mapped only after consecutive As were removed from the 3´ ends are shown in a lighter shade. Approximate region where polyadenylation takes place is indicated with a dotted arrow. (C) Average ribosome occupancy from 3´ UTRs aligned at the annotated site of polyadenylation for wild-type and _dom34_Δ strains. (D) Same as (C) except that only 99 genes with high 3´ UTR ribosome occupancy in the _dom34_Δ strain were included (>0.33 rpm and >3× as many reads as in the wild-type sample) and a broader 5´ window region was used to reveal ribosome stacking. (E) Histogram of A count on the 3´ ends of footprint reads before alignment to the genome. (Inset) Cartoon of a ribosome after a 16-nt incursion into the poly(A) tail (dashed line indicates poly(A)). (F) Normalized average ribosome occupancy from genes aligned at their stop codons for _dom34_Δ cells under normal or runoff conditions (top). Traces were normalized to the average value of ribosome density over the last 200 nt of data shown. Footprints mapping to the MFA2 gene in the _dom34_Δ strain (bottom) reveal that reads in the 3´ UTR are enriched relative to those in the ORF during starvation. (G) Examples of mapped footprints on ribosomal protein genes with identical (RPL19) or nearly identical (RPS19) ORF amino-acid sequences and very different 3´ UTR sequences. Data show substantial differences in 3´ UTR ribosome occupancy in the _dom34_Δ strain. Reads that mapped only after consecutive As were removed from the 3´ ends are shown in a lighter shade. Note that ORF reads are truncated by the vertical axis scaling and are missing in some places because reads cannot be uniquely mapped. In each case, overall ORF ribosome density differs by a factor of 1.4 between the two copies of the gene. See also Figures S4 and S5. See also Tables S1 and S2.
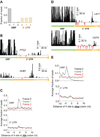
Figure 4. Many Ribosomes Do Not Translate the 3´ UTR
(A) Fraction of 28-nt reads that map (without mismatches) to each reading frame in the ORF and 3´ UTR for _dom34_Δ cells. Normalized data were averaged from ribosomes stabilized with CHX, 10 mM MgCl2, or no extra additive, but not GMP-PNP as frame was less well preserved. (B) Examples of mapped footprints from _dom34_Δ cells treated with 3-AT on genes (PTC2 and HUB1) with in-frame His codons (indicated with red arrows) in the 3´ UTR. Note that the vertical axis scaling truncates the upper range of ORF reads for PTC2. (C) Average fraction of ribosome occupancy within a 20-nt window around His codons in a given frame. Data are shown for all three reading frames in ORFs and 3´ UTRs from _dom34_Δ cells treated with 3-AT. (D) Examples of mapped footprints from _dom34_Δ cells on genes (LAT1 and OST5) with stop codons in all three reading frames of the 3´ UTR. Stop codons and their respective reading frame before the poly(A) tail are indicated (red arrows and values 0–2). Note that the vertical axis scaling truncates the upper range of ORF reads. (E) Average fraction of ribosome occupancy within a 20-nt window around the first occurrence of a stop codon in a given frame. Data are shown for all three reading frames in ORFs and 3´ UTRs in _dom34_Δ cells.
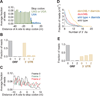
Figure 5. Ribosome Occupancy in 3´ UTR is Modulated by a Suppressor tRNA or Addition of Diamide
(A) Average fraction of ribosome occupancy in a region around the stop codon of annotated ORFs. Data taken from _ski2_Δ cells transformed with the SUP4-o tRNA. Only reads that measured 28 nt in length and mapped without mismatches are plotted. Readthrough is increased only when the stop codon is UAA. (B) Fraction of 28-nt reads that map (without mismatches) to each reading frame in the ORF and 3´ UTR for _ski2_Δ cells transformed with the SUP4-o tRNA. (C) Average fraction of ribosome occupancy within a 20-nt window around the first occurrence of a stop codon in a given frame. Data are shown only in 3´ UTRs that follow ORFs terminating with a UAA stop codon. Data from _ski2_Δ cells transformed with the SUP4-o tRNA. (D) Histogram of A count on the 3´ ends of footprint reads before alignment to the genome. Data for wild-type and _dom34_Δ yeast in the presence and absence of diamide are shown. (E) Fraction of 28-nt reads that map (without mismatches) to each reading frame in the ORF and 3´ UTR for _dom34_Δ cells treated with diamide.
Figure 6. Model for Dom34-mediated Rescue of 3´ UTR-located Ribosomes
When the ribosome reaches the 3´ end of an ORF, release factors (eRF1 and eRF3) recognize the stop codon in the A site and bind the ribosome (1). After peptide release, Rli1 typically mediates ribosome recycling (perhaps with other unknown factors) making the individual subunits available for reuse (R1). If the recycling step fails, the 80S ribosome (2) “scans” along the 3´ UTR. The ribosome then arrests upon reaching the poly(A) tail and rescue factors (Dom34 and Hbs1) bind the ribosome (3). These factors target the ribosome for recycling by Rli1, making the subunits available for reuse (R1). In some cases, the ribosome may reinitiate translation in the 3´ UTR prior to rescue.
Comment in
- Ribosome rescue, nearing the end.
Starosta AL, Wilson DN. Starosta AL, et al. Cell. 2014 Feb 27;156(5):866-7. doi: 10.1016/j.cell.2014.02.028. Cell. 2014. PMID: 24581485
Similar articles
- Ribosome rescue, nearing the end.
Starosta AL, Wilson DN. Starosta AL, et al. Cell. 2014 Feb 27;156(5):866-7. doi: 10.1016/j.cell.2014.02.028. Cell. 2014. PMID: 24581485 - Dom34-Hbs1 mediated dissociation of inactive 80S ribosomes promotes restart of translation after stress.
van den Elzen AM, Schuller A, Green R, Séraphin B. van den Elzen AM, et al. EMBO J. 2014 Feb 3;33(3):265-76. doi: 10.1002/embj.201386123. Epub 2014 Jan 14. EMBO J. 2014. PMID: 24424461 Free PMC article. - Dom34:hbs1 plays a general role in quality-control systems by dissociation of a stalled ribosome at the 3' end of aberrant mRNA.
Tsuboi T, Kuroha K, Kudo K, Makino S, Inoue E, Kashima I, Inada T. Tsuboi T, et al. Mol Cell. 2012 May 25;46(4):518-29. doi: 10.1016/j.molcel.2012.03.013. Epub 2012 Apr 11. Mol Cell. 2012. PMID: 22503425 - No-go decay: a quality control mechanism for RNA in translation.
Harigaya Y, Parker R. Harigaya Y, et al. Wiley Interdiscip Rev RNA. 2010 Jul-Aug;1(1):132-41. doi: 10.1002/wrna.17. Epub 2010 May 6. Wiley Interdiscip Rev RNA. 2010. PMID: 21956910 Review. - Ribosome pausing, arrest and rescue in bacteria and eukaryotes.
Buskirk AR, Green R. Buskirk AR, et al. Philos Trans R Soc Lond B Biol Sci. 2017 Mar 19;372(1716):20160183. doi: 10.1098/rstb.2016.0183. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28138069 Free PMC article. Review.
Cited by
- Structure of a Cytoplasmic 11-Subunit RNA Exosome Complex.
Kowalinski E, Kögel A, Ebert J, Reichelt P, Stegmann E, Habermann B, Conti E. Kowalinski E, et al. Mol Cell. 2016 Jul 7;63(1):125-34. doi: 10.1016/j.molcel.2016.05.028. Epub 2016 Jun 23. Mol Cell. 2016. PMID: 27345150 Free PMC article. - Widespread Co-translational RNA Decay Reveals Ribosome Dynamics.
Pelechano V, Wei W, Steinmetz LM. Pelechano V, et al. Cell. 2015 Jun 4;161(6):1400-12. doi: 10.1016/j.cell.2015.05.008. Cell. 2015. PMID: 26046441 Free PMC article. - Tma64/eIF2D, Tma20/MCT-1, and Tma22/DENR Recycle Post-termination 40S Subunits In Vivo.
Young DJ, Makeeva DS, Zhang F, Anisimova AS, Stolboushkina EA, Ghobakhlou F, Shatsky IN, Dmitriev SE, Hinnebusch AG, Guydosh NR. Young DJ, et al. Mol Cell. 2018 Sep 6;71(5):761-774.e5. doi: 10.1016/j.molcel.2018.07.028. Epub 2018 Aug 23. Mol Cell. 2018. PMID: 30146315 Free PMC article. - Ageing exacerbates ribosome pausing to disrupt cotranslational proteostasis.
Stein KC, Morales-Polanco F, van der Lienden J, Rainbolt TK, Frydman J. Stein KC, et al. Nature. 2022 Jan;601(7894):637-642. doi: 10.1038/s41586-021-04295-4. Epub 2022 Jan 19. Nature. 2022. PMID: 35046576 Free PMC article. - The Tomato Translational Landscape Revealed by Transcriptome Assembly and Ribosome Profiling.
Wu HL, Song G, Walley JW, Hsu PY. Wu HL, et al. Plant Physiol. 2019 Sep;181(1):367-380. doi: 10.1104/pp.19.00541. Epub 2019 Jun 27. Plant Physiol. 2019. PMID: 31248964 Free PMC article.
References
- Amrani N, Ganesan R, Kervestin S, Mangus DA, Ghosh S, Jacobson A. A faux 3'-UTR promotes aberrant termination and triggers nonsense-mediated mRNA decay. Nature. 2004;432:112–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases