Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases - PubMed (original) (raw)
Clinical Trial
Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases
Peter Gasser et al. J Nerv Ment Dis. 2014 Jul.
Free PMC article
Abstract
A double-blind, randomized, active placebo-controlled pilot study was conducted to examine safety and efficacy of lysergic acid diethylamide (LSD)-assisted psychotherapy in 12 patients with anxiety associated with life-threatening diseases. Treatment included drug-free psychotherapy sessions supplemented by two LSD-assisted psychotherapy sessions 2 to 3 weeks apart. The participants received either 200 μg of LSD (n = 8) or 20 μg of LSD with an open-label crossover to 200 μg of LSD after the initial blinded treatment was unmasked (n = 4). At the 2-month follow-up, positive trends were found via the State-Trait Anxiety Inventory (STAI) in reductions in trait anxiety (p = 0.033) with an effect size of 1.1, and state anxiety was significantly reduced (p = 0.021) with an effect size of 1.2, with no acute or chronic adverse effects persisting beyond 1 day after treatment or treatment-related serious adverse events. STAI reductions were sustained for 12 months. These results indicate that when administered safely in a methodologically rigorous medically supervised psychotherapeutic setting, LSD can reduce anxiety, suggesting that larger controlled studies are warranted.
Trial registration: ClinicalTrials.gov NCT00920387.
Figures
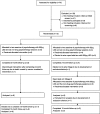
FIGURE 1
Consolidated standards of reporting trials LSD/anxiety flow diagram.
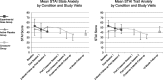
FIGURE 2
Study outcomes. State and trait anxiety scores in the LSD and placebo group. Values are mean ± SEM of changes from baseline in eight subjects in the LSD group and three subjects in the placebo group. Measures were obtained before the first treatment session (baseline), 1 week after the first treatment (post 1 LSD), 1 week after the second treatment (LSD 2), and at follow-up after 2 months. At 2 months, state anxiety scores were significantly lower in the LSD group compared with the placebo group. The crossover group (n = 3) shows a positive trend of STAI state and trait score reduction. At 12-month follow-up, the state and trait values remain stable compared with the 2-month follow-up.
Similar articles
- LSD-assisted psychotherapy for anxiety associated with a life-threatening disease: a qualitative study of acute and sustained subjective effects.
Gasser P, Kirchner K, Passie T. Gasser P, et al. J Psychopharmacol. 2015 Jan;29(1):57-68. doi: 10.1177/0269881114555249. Epub 2014 Nov 11. J Psychopharmacol. 2015. PMID: 25389218 Clinical Trial. - Lysergic Acid Diethylamide-Assisted Therapy in Patients With Anxiety With and Without a Life-Threatening Illness: A Randomized, Double-Blind, Placebo-Controlled Phase II Study.
Holze F, Gasser P, Müller F, Dolder PC, Liechti ME. Holze F, et al. Biol Psychiatry. 2023 Feb 1;93(3):215-223. doi: 10.1016/j.biopsych.2022.08.025. Epub 2022 Sep 5. Biol Psychiatry. 2023. PMID: 36266118 Clinical Trial. - MDMA-assisted psychotherapy for treatment of anxiety and other psychological distress related to life-threatening illnesses: a randomized pilot study.
Wolfson PE, Andries J, Feduccia AA, Jerome L, Wang JB, Williams E, Carlin SC, Sola E, Hamilton S, Yazar-Klosinski B, Emerson A, Mithoefer MC, Doblin R. Wolfson PE, et al. Sci Rep. 2020 Nov 24;10(1):20442. doi: 10.1038/s41598-020-75706-1. Sci Rep. 2020. PMID: 33235285 Free PMC article. Clinical Trial. - Lysergic Acid Diethylamide (LSD) for the Treatment of Anxiety Disorders: Preclinical and Clinical Evidence.
Inserra A, Piot A, De Gregorio D, Gobbi G. Inserra A, et al. CNS Drugs. 2023 Sep;37(9):733-754. doi: 10.1007/s40263-023-01008-5. Epub 2023 Aug 21. CNS Drugs. 2023. PMID: 37603260 Review. - Dark Classics in Chemical Neuroscience: Lysergic Acid Diethylamide (LSD).
Nichols DE. Nichols DE. ACS Chem Neurosci. 2018 Oct 17;9(10):2331-2343. doi: 10.1021/acschemneuro.8b00043. Epub 2018 Mar 1. ACS Chem Neurosci. 2018. PMID: 29461039 Review.
Cited by
- Self-inflicted neck wounds under influence of lysergic acid diethylamide: A case report and literature review.
Le Daré B, Gicquel T, Baert A, Morel I, Bouvet R. Le Daré B, et al. Medicine (Baltimore). 2020 Jul 2;99(27):e20868. doi: 10.1097/MD.0000000000020868. Medicine (Baltimore). 2020. PMID: 32629675 Free PMC article. - The therapeutic potential of microdosing psychedelics in depression.
Kuypers KPC. Kuypers KPC. Ther Adv Psychopharmacol. 2020 Aug 27;10:2045125320950567. doi: 10.1177/2045125320950567. eCollection 2020. Ther Adv Psychopharmacol. 2020. PMID: 32922736 Free PMC article. Review. - Acute and Sustained Reductions in Loss of Meaning and Suicidal Ideation Following Psilocybin-Assisted Psychotherapy for Psychiatric and Existential Distress in Life-Threatening Cancer.
Ross S, Agin-Liebes G, Lo S, Zeifman RJ, Ghazal L, Benville J, Franco Corso S, Bjerre Real C, Guss J, Bossis A, Mennenga SE. Ross S, et al. ACS Pharmacol Transl Sci. 2021 Mar 18;4(2):553-562. doi: 10.1021/acsptsci.1c00020. eCollection 2021 Apr 9. ACS Pharmacol Transl Sci. 2021. PMID: 33860185 Free PMC article. - LSD-assisted therapy in patients with anxiety: open-label prospective 12-month follow-up.
Holze F, Gasser P, Müller F, Strebel M, Liechti ME. Holze F, et al. Br J Psychiatry. 2024 Sep;225(3):362-370. doi: 10.1192/bjp.2024.99. Br J Psychiatry. 2024. PMID: 39078038 Free PMC article. Clinical Trial. - Psychedelics and fNIRS neuroimaging: exploring new opportunities.
Scholkmann F, Vollenweider FX. Scholkmann F, et al. Neurophotonics. 2023 Jan;10(1):013506. doi: 10.1117/1.NPh.10.1.013506. Epub 2022 Dec 2. Neurophotonics. 2023. PMID: 36474478 Free PMC article.
References
- Aaronson N, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Fletchner H, Fleischman SB, de Haes JC. (1993). The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 85: 365– 376 - PubMed
- Abramson H. (Ed) (1967). The use of LSD in psychotherapy and alcoholism. New York: Bobbs-Merrill
- Bonson KR, Buckholtz JW, Murphy DL. (1996). Chronic administration of serotonergic antidepressants attenuates the subjective effects of LSD in humans. Neuropsychopharmacology. 14: 425– 436 - PubMed
- Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, Tyacke RJ, Leech R, Malizia AL, Murphy K, Hobden P, Evans J, Feilding A, Wise RG, Nutt DJ. (2012). Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A. 109: 2138– 2143 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical