Neutral lipid stores and lipase PNPLA5 contribute to autophagosome biogenesis - PubMed (original) (raw)
Neutral lipid stores and lipase PNPLA5 contribute to autophagosome biogenesis
Nicolas Dupont et al. Curr Biol. 2014.
Abstract
Background: Autophagy is a fundamental cell biological process whereby eukaryotic cells form membranes in the cytoplasm to sequester diverse intracellular targets. Although significant progress has been made in understanding the origins of autophagosomal organelles, the source of lipids that support autophagic membrane formation remain an important open question.
Results: Here we show that lipid droplets as cellular stores of neutral lipids including triglycerides contribute to autophagic initiation. Lipid droplets, as previously shown, were consumed upon induction of autophagy by starvation. However, inhibition of autophagic maturation by blocking acidification or using dominant negative Atg4(C74A) that prohibits autophagosomal closure did not prevent disappearance of lipid droplets. Thus, lipid droplets continued to be utilized upon induction of autophagy, but not as autophagic substrates in a process referred to as lipophagy. We considered an alternative model whereby lipid droplets were consumed not as a part of lipophagy, but as a potential contributing source to the biogenesis of lipid precursors for nascent autophagosomes. We carried out a screen for a potential link between triglyceride mobilization and autophagy and identified a neutral lipase, PNPLA5, as being required for efficient autophagy. PNPLA5, which localized to lipid droplets, was needed for optimal initiation of autophagy. PNPLA5 was required for autophagy of diverse substrates, including degradation of autophagic adaptors, bulk proteolysis, mitochondrial quantity control, and microbial clearance.
Conclusions: Lipid droplets contribute to autophagic capacity by enhancing it in a process dependent on PNPLA5. Thus, neutral lipid stores are mobilized during autophagy to support autophagic membrane formation.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
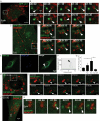
Figure 1. Preformed lipid droplets enhance starvation-induced autophagy
(A) and (B) HeLa cells stably expressing mRFP-GFP-LC3 were treated for 20 h with BSA alone (BSA) or with BSA-oleic acid (OA; 500 μM OA) and starved (Starv) in EBSS for 90 min or incubated in full medium (Full). (A) Visualization of lipid droplet accumulation. Lipid droplets were stained with Bodipy 493/503. (B) Confocal images of samples (for images in high content acquisition mode see Figure S1). (C) and (D) Number of GFP+ puncta (C) and RFP+ puncta (D) per cell were quantified by high content image acquisition and analysis. Data: means ± s.e. (n=3, where n represents separate experiments; each experimental point in separate experiments contained >500 cells identified by the program as valid primary objects); *, p<0.05 (t-test). (E,F) HeLa cells treated with OA as in A–D with or without bafilomycin A1 (BafA1); LC3-II/actin ratios determined by immunoblotting (E) and densitometry (F). Immunobloting data: means ± s.e., *, p<0.05 (t-test).
Figure. 2. Imaging analysis of dynamic interactions between lipid droplets and LC3, WIPI2D and a diacylglycerol GFP probes
(A,B) Still frames from time lapse imaging of GFP-LC3 expressing HeLa cells (Movie S1). HeLa cells expressing GFP-LC3 (green) treated for 20 h with 500 μM OA (BSA-oleic acid), LDs (red) labeled with LipidTox Farred, and live imaging initiated in starvation (EBSS) medium. Time, min:sec. Rectangle in A, area in time-lapse still frames in B. Arrowheads, a GFP-LC3 positive structure (green) interacting with a lipid droplet (red). (C,D) Still frames from intravital imaging of intact liver from GFP-LC3 mice (Movie S2). Time, h:min:sec.. Arrowheads, a GFP-LC3 positive structure (green) interacting with a lipid droplet (red). (E–G) Confocal microscopy analysis of U2OS cells stably expressing GFP WIPI-2D. Lipid droplets were visualized (blue channel) with LipidTox DeepRed. Cells were treated as in A, starved for 2 h and then fixed. Arrowheads, recruitment of WIPI2D to lipid droplets. (F) Two-fluorescence channel line tracings corresponding to dashed lines in images to the left. (G) WIPI-2D positive LDs were quantified by Image J. Cells were pre-treated for 20 h with 500 μM BSA-oleic acid (OA) then starved (OA Starv) or not (OA Full) for 2 h in the absence or presence of 3-MA treatment (OA Starv3MA). Data means ± s.e. (n≥3); *, p<0.05. (H,I) Still frames from live imaging of U2OS cells stably expressing GFP-WIPI2D (Movies S3 and S4). Cells were treated as in A. Time, h:min:sec. Rectangle in H, area in still frames in I. Arrowheads, an emerging GFP-WIPI2D positive (green) structure juxtaposed to an LD, enlarging in size with time. (J,K) Still frames from live imaging of GFP-DAG expressing HeLa cells (Movies S5 and S6). HeLa cells expressing NES-GFP-DAG (green) were treated as in A. Time, min:sec. Rectangle in J, still frames in K. Arrowheads, a GFP-DAG positive (green) structure emerging juxtaposed to LD.
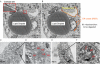
Figure. 3. Ultrastructural analysis of associations between lipid droplets and phagophores
(A–F) U2OS cells were treated for 24 h with 500 μM BSA-oleic acid and processed for electron microscopy (cumulative analysis in Table S1). (A,D) Examples of autophagic phagophores (isolation membranes) in the proximity of LDs. M, mitochondrion; RER, rough endoplasmic reticulum. Enlarged detail in A, membranous anastomosis between a lipid droplet and a phagophore. (E,F) Examples of LDs, including LDs as substrate for autophagy (lipophagy).
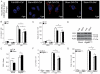
Figure. 4. Lipid droplets are consumed during autophagic induction independently of autophagosomal closure and autophagic maturation
(A) Confocal microscopy image of HeLa cells treated as in Figs 1 and 2 with or without Bafilomycin A1 (Baf) to inhibit autophagic degradation. Cells were stained with Hoechst 33342 (nuclei; blue) and Bodipy 493/503 (lipid droplets; red). Confocal images illustrate high content analysis in (B,C) carried out in epifluoresce mode (Figure S5A). Lipid droplets (LD) number (B) and total LD area (C) were quantified by high content image acquisition and analysis. (D,E) Stable 3T3 cells expressing ATG4B or mStrawberry-ATG4BC74A were treated as in A. p62/actin ratios were determined by immunoblotting (D) and densitometry (E). Immunoblot data, means ± s.e., (n≥3); *, p<0.05. (F,G) Stable 3T3 cells expressing ATG4B or mStrawberry-ATG4BC74A were treated, lipid droplets stained as in A, and analyzed as in B and C. All high content analysis data, means ± s.e (n=3, where n represents separate experiments; each experimental point in separate experiments contained >500 cells identified by the program as valid primary objects); *, p<0.05 †, p≥0.05 (t-test).
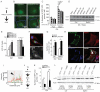
Figure 5. Screen for triglyceride metabolism factors identifies PNPLA5 and CPT1 as positive regulators of autophagy
(A) Triglyceride (TG) catabolic pathway (lipolysis), PNPLAs (1/2/3/4/5), PNPLAs, patatin-like phospholipase domain-containing proteins 1 through 5. (B,C) HeLa cells stably expressing mRFP-GFP-LC3 were transfected twice with scrambled (Scr) control siRNA or siRNAs against PNPLAs. Cells were treated as in Fig. 1. (C) high content image acquisition and analysis. (D,E) Effect of PNPLAs on autophagy induction by measuring LC3-II levels by immunoblotting and densitometry. HeLa cells transfected twice with siRNAs (PNPLA 2,3,5) or scrambled (Scr) were treated as in A with or without Bafilomycin A1 (Baf). Immunoblotting data, means ± s.e. (n≥3); *, p<0.05. (F,G) PNPLA5 overexpression effects on endogenous LC3 puncta. HeLa cells were transfected with GFP or PNPLA5-GFP, treated 20 h with 500 μM BSA-Oleic Acid (OA) and starved for 2 h with or without Bafilomycin A1 (Baf). Endogenous LC3 was stained by immunofluorescence and LC3 dots were quantified within GFP positive cells (as illustrated in fluorescent images in F) by high content image acquisition and analysis in G. (H,I) Confocal microscopy of HeLa cells transfected with PNPLA5-GFP expression plasmid (green cell), ATG16L1 (red) and lipid droplets (LD, LipidTox DeepRed, blue channel). Cells were transfected with PNPLA5-GFP expressing plasmid, treated for 20 h with 500 μM BSA-Oleic Acid (OA), fixed, and lipid droplets stained with LipidTox DeepRed and immunostained for ATG16L1. Arrowhead, colocalization of PNPLA5GFP, ATG16L1 on lipid droplets; dashed line, two-fluorescence channel line tracing shown in panel I. (J) Scheme, enzymes involved in the phospholipid synthesis pathway. (K) HeLa cells stably expressing mRFP-GFP-LC3 were transfected twice with scramble control (Scr) or CPT1 siRNAs. After 24 h, cells were treated for 20 h with 500 μM BSA-Oleic Acid (OA) and starved for 90 min. GFP+ puncta per cell were quantified by high content image acquisition and analysis. (L) Effect of CPT1 on autophagy induction by determininig LC3-II levels. HeLa cells were transfected with siRNAs against CPT1 or scrambled (Scr) control. After 24 h, cells were cotransfected with siRNAs against CPT1 or scrambled (Scr) control and with plasmids expressing wild-type (WT) CPT1-mCherry or mutant CPT1-mCherry T468C (SiRNA resistant construct). Cells were then treated 20 h with 500 μM BSA-Oleic Acid (OA) and starved for 2 h with or without Bafilomycin A1 (Baf) and subjected to immunoblot analysis (see Supplementary Figure S7H for LC3-II/actin ratios determined by densitometry).
Figure. 6. Localization analysis of DAG and ATG16L1 juxtaposed to lipid droplets upon overexpression of mutant ATG4BC74A
(A–G) Diacylglycerol (DAG, green; revealed by the NES-GFP-DAG probe) and ATG16L1 (red) relative to lipid droplets (LD, blue). 3T3 cells expressing ATG4B (A; Mock) or ATG4BC74A (B) proteins were transfected with a plasmid expressing NES-GFP-DAG, treated for 20 h with 500 μM BSA-Oleic Acid, and starved in EBSS for 150 minutes. ATG16L1, immunostatining. Arrows, overlap between ATG16L1 and DAG signals juxtaposed to LD. (C,D) Enlarged area (dashed box in B) and fluorescence line tracings. (E) Quantification of the recruitment of GFP-DAG probe to LDs. DAG positive LDs were quantified by using SlideBook morphometric analysis software (details in Materials and Methods). 3T3 cells expressing ATG4B (Mock) or ATG4BC74A proteins were processed as in A. (F). ATG16L1 positive LDs were quantified using SlideBook morphometric analysis software in 3T3 cells expressing ATG4B (Mock) or ATG4BC74A processed as in A and B. Data, means ± s.e. (n≥3); *, p<0.05. (G) Pearson's coefficients for DAG and Atg16L1 surrounding the droplets (SlideBook morphometry; 3 independent experiments with 5 fields per experiment). Data, means ± s.e. (n≥3); *, p<0.05
Figure. 7. PNPLA5 is required for efficient autophagy of diverse substrates
(A,B) HeLa cells were transfected twice with PNPLA5 or scramble (Scr) siRNA control, treated for 20 h with BSA or with 500 μM BSA-Oleic Acid (OA) and starved (Starv) or not (Full) for 2 h, lipid droplets stained with Bodipy 493/503 (illustrated in Figure S6) and quontified by high content imaging acquisition and analysis. (C–E) HeLa cells transfected and treated as in A; total immunofluorescence intensity of endogenous p62 was quantified in GFP-positive cells by high content image acquisition and analysis (C). (D,E) p62/actin ratios determined by immunoblotting and densitometry. (F) Proteolysis of proteins in HeLa cells. HeLa transfected and treated as in A, in media containing [3H] leucine, were starved or not with or without Bafilomycin A1 (Baf) for 90 min. Leucine release was calculated from radioactivity in the tricarboxylic acid-soluble form relative to total cell radioactivity. (G) Lipid droplet targeting motif of PNPLA5, Red dots, residues that have been mutated to Ala (RSRRLV changed to ASAALV). (H) Proteolysis of long-lived proteins in HeLa cels were transfected with plasmids expressing wild-type (WT) GFP-tagged or ASAALV PNPLA5 proteins and treated 20 h with 500 μM BSA-Oleic Acid (OA) in media containing [14C] valine. Y-axis, valine release from stable proteins. (I) CDP-alcohol phosphotransferase catalytic motif in CPT1. Red dot, Cys-to-Ala mutation. (J) HeLa cells were transfected with plasmids expressing wild-type (WT) mCherry-tagged or CPT1Gly128Ala mutant CPT1 proteins and processed for proteolysis of long-lived proteins as in (H). (K,L) Flow cytometry analysis of cellular mitochondrial content. HeLa were treated as in A (full medium) and stained with MitoTracker Green. (K) histograms; (L) average mean fluorescence intensity (MFI) of MitoTracker Green per cell. Data, means ± s.e. (n≥3); *, p<0.05. (M) Analysis of the role of PNPLA5 in autophagic killing of BCG. RAW 264.7 macrophages were transfected twice with PNPLA5 siRNAs or scramble (Scr) control. Cells were then treated 20 h with 250 μM BSA-Oleic Acid (OA) and infected the day after with BCG. Autophagy was induced 4 h by starvation (Starv). BCG survival, % of control BCG CFU. Data, means ± s.e.m. *, p<0.05. (N) Model for how lipid droplets contribute to autophagosome biogenesis (see discussion).
Similar articles
- Autophagy and Lipid Droplets in the Liver.
Martinez-Lopez N, Singh R. Martinez-Lopez N, et al. Annu Rev Nutr. 2015;35:215-37. doi: 10.1146/annurev-nutr-071813-105336. Epub 2015 May 6. Annu Rev Nutr. 2015. PMID: 26076903 Free PMC article. Review. - Lipid droplets and their component triglycerides and steryl esters regulate autophagosome biogenesis.
Shpilka T, Welter E, Borovsky N, Amar N, Mari M, Reggiori F, Elazar Z. Shpilka T, et al. EMBO J. 2015 Aug 13;34(16):2117-31. doi: 10.15252/embj.201490315. Epub 2015 Jul 10. EMBO J. 2015. PMID: 26162625 Free PMC article. - Recycling the danger via lipid droplet biogenesis after autophagy.
Li Y, Zong WX, Ding WX. Li Y, et al. Autophagy. 2017;13(11):1995-1997. doi: 10.1080/15548627.2017.1371394. Epub 2017 Oct 4. Autophagy. 2017. PMID: 28873005 Free PMC article. - Autophagy regulates lipid metabolism.
Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Singh R, et al. Nature. 2009 Apr 30;458(7242):1131-5. doi: 10.1038/nature07976. Epub 2009 Apr 1. Nature. 2009. PMID: 19339967 Free PMC article. - Regulation of lipid stores and metabolism by lipophagy.
Liu K, Czaja MJ. Liu K, et al. Cell Death Differ. 2013 Jan;20(1):3-11. doi: 10.1038/cdd.2012.63. Epub 2012 May 18. Cell Death Differ. 2013. PMID: 22595754 Free PMC article. Review.
Cited by
- Phospholipid Scramblase Activity of VDAC Dimers: New Implications for Cell Death, Autophagy and Ageing.
Rockenfeller P. Rockenfeller P. Biomolecules. 2024 Sep 26;14(10):1218. doi: 10.3390/biom14101218. Biomolecules. 2024. PMID: 39456151 Free PMC article. Review. - Autophagy and Lipid Droplets in the Liver.
Martinez-Lopez N, Singh R. Martinez-Lopez N, et al. Annu Rev Nutr. 2015;35:215-37. doi: 10.1146/annurev-nutr-071813-105336. Epub 2015 May 6. Annu Rev Nutr. 2015. PMID: 26076903 Free PMC article. Review. - Phospholipid imbalance impairs autophagosome completion.
Polyansky A, Shatz O, Fraiberg M, Shimoni E, Dadosh T, Mari M, Reggiori FM, Qin C, Han X, Elazar Z. Polyansky A, et al. EMBO J. 2022 Dec 1;41(23):e110771. doi: 10.15252/embj.2022110771. Epub 2022 Oct 27. EMBO J. 2022. PMID: 36300838 Free PMC article. - Emerging Roles of Lipophagy in Health and Disease.
Kounakis K, Chaniotakis M, Markaki M, Tavernarakis N. Kounakis K, et al. Front Cell Dev Biol. 2019 Sep 10;7:185. doi: 10.3389/fcell.2019.00185. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31552248 Free PMC article. Review. - De novo lipogenesis fuels adipocyte autophagosome and lysosome membrane dynamics.
Rowland LA, Guilherme A, Henriques F, DiMarzio C, Munroe S, Wetoska N, Kelly M, Reddig K, Hendricks G, Pan M, Han X, Ilkayeva OR, Newgard CB, Czech MP. Rowland LA, et al. Nat Commun. 2023 Mar 13;14(1):1362. doi: 10.1038/s41467-023-37016-8. Nat Commun. 2023. PMID: 36914626 Free PMC article.
References
- Mizushima N, Yoshimori T, Ohsumi Y. The role of atg proteins in autophagosome formation. Annual review of cell and developmental biology. 2011;27:107–132. - PubMed
- Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P. Distinct classes of phosphatidylinositol 3'-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem. 2000;275:992–998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases