A systematic development process for patient decision aids - PubMed (original) (raw)
Review
A systematic development process for patient decision aids
Angela Coulter et al. BMC Med Inform Decis Mak. 2013.
Abstract
Background: The original version of the International Patient Decision Aid Standards (IPDAS) recommended that patient decision aids (PtDAs) should be carefully developed, user-tested and open to scrutiny, with a well-documented and systematically applied development process. We carried out a review to check the relevance and scope of this quality dimension and, if necessary, to update it.
Methods: Our review drew on three sources: a) published papers describing PtDAs evaluated in randomised controlled trials and included in the most recent Cochrane Collaboration review; b) linked papers cited in the trial reports that described how the PtDAs had been developed; and c) papers and web reports outlining the development process used by organisations experienced in developing multiple PtDAs. We then developed an extended model of the development process indicating the various steps on which documentation is required, as well as a checklist to assess the frequency with which each of the elements was publicly reported.
Results: Key features common to all patient decision aid (PtDA) development processes include: scoping and design; development of a prototype; 'alpha' testing with patients and clinicians in an iterative process; 'beta' testing in 'real life' conditions (field tests); and production of a final version for use and/or further evaluation. Only about half of the published reports on the development of PtDAs that we reviewed appear to have been field tested with patients, and even fewer had been reviewed or tested by clinicians not involved in the development process. Very few described a distribution strategy, and surprisingly few (17%) described a method for reviewing and synthesizing the clinical evidence. We describe a model development process that includes all the original elements of the original IPDAS criterion, expanded to include consideration of format and distribution plans as well as prototype development.
Conclusions: The case for including each of the elements outlined in our model development process is pragmatic rather than evidence-based. Optimal methods for ensuring that each stage of the process is carried out effectively require further development and testing.
Figures
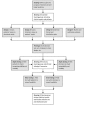
Figure 1
Model Development Process for Decision Aids
Similar articles
- Do patient decision aids meet effectiveness criteria of the international patient decision aid standards collaboration? A systematic review and meta-analysis.
O'Connor AM, Bennett C, Stacey D, Barry MJ, Col NF, Eden KB, Entwistle V, Fiset V, Holmes-Rovner M, Khangura S, Llewellyn-Thomas H, Rovner DR. O'Connor AM, et al. Med Decis Making. 2007 Sep-Oct;27(5):554-74. doi: 10.1177/0272989X07307319. Epub 2007 Sep 14. Med Decis Making. 2007. PMID: 17873255 Review. - Providing Balanced Information about Options in Patient Decision Aids: An Update from the International Patient Decision Aid Standards.
Martin RW, Brogård Andersen S, O'Brien MA, Bravo P, Hoffmann T, Olling K, Shepherd HL, Dankl K, Stacey D, Dahl Steffensen K. Martin RW, et al. Med Decis Making. 2021 Oct;41(7):780-800. doi: 10.1177/0272989X211021397. Epub 2021 Jul 1. Med Decis Making. 2021. PMID: 34196241 - Patient decision aids for patients with differentiated thyroid carcinoma: development process and alpha and beta testing.
Koot A, Hermens R, Ottevanger P, Netea-Maier R, Stalmeier P; COMBO study group. Koot A, et al. Front Endocrinol (Lausanne). 2023 May 31;14:1162537. doi: 10.3389/fendo.2023.1162537. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37324263 Free PMC article. - Guidance and/or Decision Coaching with Patient Decision Aids: Scoping Reviews to Inform the International Patient Decision Aid Standards (IPDAS).
Rahn AC, Jull J, Boland L, Finderup J, Loiselle MC, Smith M, Köpke S, Stacey D. Rahn AC, et al. Med Decis Making. 2021 Oct;41(7):938-953. doi: 10.1177/0272989X21997330. Epub 2021 Mar 24. Med Decis Making. 2021. PMID: 33759626 Review. - A Systematic Review and Meta-Analysis of Patient Decision Aids for Socially Disadvantaged Populations: Update from the International Patient Decision Aid Standards (IPDAS).
Yen RW, Smith J, Engel J, Muscat DM, Smith SK, Mancini J, Perestelo-Pérez L, Elwyn G, O'Malley AJ, Leyenaar JK, Mac O, Cadet T, Giguere A, Housten AJ, Langford A, McCaffery K, Durand MA. Yen RW, et al. Med Decis Making. 2021 Oct;41(7):870-896. doi: 10.1177/0272989X211020317. Epub 2021 Jun 21. Med Decis Making. 2021. PMID: 34151614 Free PMC article.
Cited by
- Improving patient-provider communication about chronic pain: development and feasibility testing of a shared decision-making tool.
Col N, Hull S, Springmann V, Ngo L, Merritt E, Gold S, Sprintz M, Genova N, Nesin N, Tierman B, Sanfilippo F, Entel R, Pbert L. Col N, et al. BMC Med Inform Decis Mak. 2020 Oct 17;20(1):267. doi: 10.1186/s12911-020-01279-8. BMC Med Inform Decis Mak. 2020. PMID: 33069228 Free PMC article. - Increasing Readiness for Early Integrated Palliative Oncology Care: Development and Initial Evaluation of the EMPOWER 2 Intervention.
Perry LM, Sartor O, Malhotra S, Alonzi S, Kim S, Voss HM, Rogers JL, Robinson W, Harris K, Shank J, Morrison DG, Lewson AB, Fuloria J, Miele L, Lewis B, Mossman B, Hoerger M. Perry LM, et al. J Pain Symptom Manage. 2021 Nov;62(5):987-996. doi: 10.1016/j.jpainsymman.2021.03.027. Epub 2021 Apr 20. J Pain Symptom Manage. 2021. PMID: 33864847 Free PMC article. Clinical Trial. - A randomized controlled trial on the effects of decision aids for choosing discharge destinations of older stroke patients.
Aoki Y, Nakayama K, Yonekura Y. Aoki Y, et al. PLoS One. 2024 Jan 25;19(1):e0272115. doi: 10.1371/journal.pone.0272115. eCollection 2024. PLoS One. 2024. PMID: 38271437 Free PMC article. Clinical Trial. - Evaluation of a patient decision aid for initiating disease modifying anti-rheumatic drugs.
Nota I, Drossaert CH, Taal E, Vonkeman HE, Haagsma CJ, van de Laar MA. Nota I, et al. Arthritis Res Ther. 2016 Oct 28;18(1):252. doi: 10.1186/s13075-016-1138-3. Arthritis Res Ther. 2016. PMID: 27793171 Free PMC article. - Continuous adaptation of conversation aids for uterine fibroids treatment options in a four-year multi-center implementation project.
Schubbe D, Durand MA, Forcino RC, Engel J, Tomaino M, Adams-Foster M, Bacon C, Mulligan CC, Venable S, Foster T, Barr PJ, Anchan RM, Laughlin-Tommaso S, Lindholm A, Seshan M, Gargiulo RM, Stephenson P, George K, Ajao M, Madden T, Banks E, Gargiulo AR, O'Malley J, van den Muijsenbergh M, Aarts JWM, Elwyn G. Schubbe D, et al. BMC Med Inform Decis Mak. 2024 Sep 30;24(1):277. doi: 10.1186/s12911-024-02637-6. BMC Med Inform Decis Mak. 2024. PMID: 39350254 Free PMC article.
References
- Elwyn G, O'Connor A, Stacey D, Volk R, Edwards A, Coulter A, Thomson R, Barratt A, Barry M, Bernstein S, Butow P, Clarke A, Entwistle V, Feldman-Stewart D, Holmes-Rovner M, Llewellyn-Thomas H, Moumjid N, Mulley A, Ruland C, Sepucha K, Sykes A, Whelan T. International Patient Decision Aids Standards (IPDAS) Collaboration. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;13(7565):417. doi: 10.1136/bmj.38926.629329.AE. - DOI - PMC - PubMed
- O'Connor AM, Bennett CL, Stacey D, Barry M, Col NF, Eden KB, Entwistle VA, Fiset V, Holmes-Rovner M, Khangura S, Llewellyn-Thomas H, Rovner D. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2009. p. CD001431. - PubMed
- Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes-Rovner M, Llewellyn-Thomas H, Lyddiatt A, Légaré F, Thomson R. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;13:CD001431. - PubMed
- Kasper JF, Mulley AG Jr., Wennberg JE. Developing shared decision-making programs to improve the quality of health care. QRB Qual Rev Bull. 1992;13(6):183–190. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources