MiR-29b inhibits collagen maturation in hepatic stellate cells through down-regulating the expression of HSP47 and lysyl oxidase - PubMed (original) (raw)
MiR-29b inhibits collagen maturation in hepatic stellate cells through down-regulating the expression of HSP47 and lysyl oxidase
Yifei Zhang et al. Biochem Biophys Res Commun. 2014.
Abstract
Altered expression of miR-29b is implicated in the pathogenesis and progression of liver fibrosis. We and others previously demonstrated that miR-29b down-regulates the expression of several extracellular-matrix (ECM) genes including Col 1A1, Col 3A1 and Elastin via directly targeting their 3'-UTRs. However, whether or not miR-29b plays a role in the post-translational regulation of ECM biosynthesis has not been reported. Heat shock protein 47 (HSP47) and lysyl oxidase (LOX) are known to be essential for ECM maturation. In this study we have demonstrated that expression of HSP47 and LOX was significantly up-regulated in culture-activated primary rat hepatic stellate cells (HSCs), TGF-β stimulated LX-2 cells and liver tissue of CCl4-treated mice, which was accompanied by a decrease of miR-29b level. In addition, over-expression of miR-29b in LX-2 cells resulted in significant inhibition on HSP47 and LOX expression. Mechanistically, miR-29b inhibited the expression of a reporter gene that contains the respective full-length 3'-UTR from HSP47 and LOX gene, and this inhibitory effect was abolished by the deletion of a putative miR-29b targeting sequence from the 3'-UTRs. Transfection of LX-2 cells with miR-29b led to abnormal collagen structure as shown by electron-microscopy, presumably through down-regulation of the expression of molecules involved in ECM maturation including HSP47 and LOX. These results demonstrated that miR-29b is involved in regulating the post-translational processing of ECM and fibril formation.
Keywords: Extracellular matrix; Fibrosis; Heat shock protein 47; Lysyl oxidase; miR-29b.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
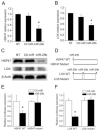
Fig. 1. Expression of HSP47, LOX and miR-29b in mouse liver with CCl4-induced fibrosis, culture-activated rat HSCs and TGF-β treated LX-2 cells
CD-1 mice were treated with corn oil or CCl4 for 6 weeks (A-B). Quiescent and activated HSCs of rat were isolated and harvested as described in the Materials and methods (C-D). LX-2 cells were treated with TGF-β (5ng/ml) and harvested at 24h after treatment (E). Quantitative PCR was conducted to detect the expression levels of HSP47 and LOX mRNA and miR-29b. Gene expression level was normalized against the control groups, and data represent quantification of four independent experiments, *P < 0.05 (A, C and E). Western blots were conducted to detect the protein expression levels of HSP47 and LOX (B and D).
Fig. 2. miR-29b decreased the expression of HSP47 and LOX by directly interacting with the 3-‘UTR of their mRNAs
Cells were transfected with either non-specific control miRNA or miR-29b at a concentration of 50 nM. The mRNA expression levels of HSP47 (A) and LOX (B) were analyzed by qRT-PCR at 24 h post-transfection. Western blots were conducted to detect the HSP47 and LOX expression at 48 h post-transfection (C). (D) Scheme of wild-type and mutant 3′-UTRs of human HSP47 and LOX. Wild type 3′-UTRs include the putative binding sites highlighted. Deletion mutant eliminates the putative binding sites. (E) LX-2 cells were transfected with a luciferase construct with wildtype or mutant HSP47-3′-UTR in the presence of 50 nM miR-29b or non-specific control miRNA. Luciferase assay was performed 24 h post-transfection. (F) The same experiment with LOX 3′-UTR luciferase vectors as in (E), miR concentration=10nM. Luciferase activity was normalized against the control groups. Data shown in the panels represent means± (SD) of the fold increase over the control. N = 3. *P < 0.05 (vs. control miRNA).
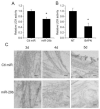
Fig. 3. Transfection of LX-2 cells with miR-29b affects their extracellular LOX activity and morphology of extracellular fibrils
(A) Extracellular LOX enzyme activity was significantly reduced in the supernatant of miR-29b transfected LX-2 cells 72 h post-transfection compared with that of control miRNA transfected cells. (B) LOX activity in the conditioned media was significantly inhibited by 100 μM BAPN. Data represent mean ± SD, n = 3. (*P < 0.05). (C) LX-2 cells were transfected with control miRNA or miR-29b. The extracellular fibrils were observed by Transmission Electron Microscopy at 3 d, 4 d and 5 d after the cells became confluent. Bar, 100 nm.
Similar articles
- Overexpression of miR-29b reduces collagen biosynthesis by inhibiting heat shock protein 47 during skin wound healing.
Zhu Y, Li Z, Wang Y, Li L, Wang D, Zhang W, Liu L, Jiang H, Yang J, Cheng J. Zhu Y, et al. Transl Res. 2016 Dec;178:38-53.e6. doi: 10.1016/j.trsl.2016.07.001. Epub 2016 Jul 15. Transl Res. 2016. PMID: 27477081 - TRAIL regulates collagen production through HSF1-dependent Hsp47 expression in activated hepatic stellate cells.
Park SJ, Sohn HY, Park SI. Park SJ, et al. Cell Signal. 2013 Jul;25(7):1635-43. doi: 10.1016/j.cellsig.2013.04.001. Epub 2013 Apr 12. Cell Signal. 2013. PMID: 23587601 - microRNA-29b prevents liver fibrosis by attenuating hepatic stellate cell activation and inducing apoptosis through targeting PI3K/AKT pathway.
Wang J, Chu ES, Chen HY, Man K, Go MY, Huang XR, Lan HY, Sung JJ, Yu J. Wang J, et al. Oncotarget. 2015 Mar 30;6(9):7325-38. doi: 10.18632/oncotarget.2621. Oncotarget. 2015. PMID: 25356754 Free PMC article. - [Therapeutic strategy for fibrotic diseases by regulating the expression of collagen-specific molecular chaperone HSP47].
Nagata K. Nagata K. Nihon Yakurigaku Zasshi. 2003 Jan;121(1):4-14. doi: 10.1254/fpj.121.4. Nihon Yakurigaku Zasshi. 2003. PMID: 12617032 Review. Japanese. - The heat shock protein 47 as a potential biomarker and a therapeutic agent in cancer research.
Duarte BDP, Bonatto D. Duarte BDP, et al. J Cancer Res Clin Oncol. 2018 Dec;144(12):2319-2328. doi: 10.1007/s00432-018-2739-9. Epub 2018 Aug 20. J Cancer Res Clin Oncol. 2018. PMID: 30128672 Review.
Cited by
- Deciphering Collagen Phenotype Dynamics Regulators: Insights from In-Silico Analysis.
Rajalekshmi R, Rai V, Agrawal DK. Rajalekshmi R, et al. J Bioinform Syst Biol. 2024;7(3):169-181. doi: 10.26502/jbsb.5107089. Epub 2024 Sep 19. J Bioinform Syst Biol. 2024. PMID: 39484658 Free PMC article. - Matrix Mechanics as Regulatory Factors and Therapeutic Targets in Hepatic Fibrosis.
Chen G, Xia B, Fu Q, Huang X, Wang F, Chen Z, Lv Y. Chen G, et al. Int J Biol Sci. 2019 Sep 7;15(12):2509-2521. doi: 10.7150/ijbs.37500. eCollection 2019. Int J Biol Sci. 2019. PMID: 31754325 Free PMC article. Review. - Recent Advancements in Antifibrotic Therapies for Regression of Liver Fibrosis.
Jangra A, Kothari A, Sarma P, Medhi B, Omar BJ, Kaushal K. Jangra A, et al. Cells. 2022 Apr 29;11(9):1500. doi: 10.3390/cells11091500. Cells. 2022. PMID: 35563807 Free PMC article. Review. - Circulating microRNAs as non-invasive biomarkers for hepatitis B virus liver fibrosis.
Iacob DG, Rosca A, Ruta SM. Iacob DG, et al. World J Gastroenterol. 2020 Mar 21;26(11):1113-1127. doi: 10.3748/wjg.v26.i11.1113. World J Gastroenterol. 2020. PMID: 32231417 Free PMC article. Review. - Hepatic Stellate Cells and microRNAs in Pathogenesis of Liver Fibrosis.
Kitano M, Bloomston PM. Kitano M, et al. J Clin Med. 2016 Mar 16;5(3):38. doi: 10.3390/jcm5030038. J Clin Med. 2016. PMID: 26999230 Free PMC article. Review.
References
- Olaso E, Friedman SL. Molecular regulation of hepatic fibrogenesis. J Hepatol. 1998;29:836–847. - PubMed
- Nagata K. HSP47: A collagen-specific molecular chaperone. Trends Biochem Sci. 1996;21:23–26. - PubMed
- Hosokawa N, Hohenadl C, Satoh M, Kuhn K, Nagata K. HSP47, a collagen-specific molecular chaperone, delays the secretion of type III procollagen transfected in human embryonic kidney cell line 293: A possible role for HSP47 in collagen modification. J Biochem. 1998;124:654–662. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous