Mitochondrial trafficking and anchoring in neurons: New insight and implications - PubMed (original) (raw)
Review
Mitochondrial trafficking and anchoring in neurons: New insight and implications
Zu-Hang Sheng. J Cell Biol. 2014.
Abstract
Mitochondria are essential organelles for neuronal growth, survival, and function. Neurons use specialized mechanisms to drive mitochondria transport and to anchor them in axons and at synapses. Stationary mitochondria buffer intracellular Ca(2+) and serve as a local energy source by supplying ATP. The balance between motile and stationary mitochondria responds quickly to changes in axonal and synaptic physiology. Defects in mitochondrial transport are implicated in the pathogenesis of several major neurological disorders. Recent work has provided new insight in the regulation of microtubule-based mitochondrial trafficking and anchoring, and on how mitochondrial motility influences neuron growth, synaptic function, and mitophagy.
Figures
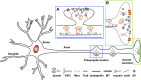
Figure 1.
Mitochondrial trafficking and anchoring in neurons. Due to complex structural features, neurons require specialized mechanisms trafficking mitochondria to their distal destinations and anchoring them in regions where metabolic and calcium homeostatic capacity is in a high demand. The figure highlights transporting mitochondria to a presynaptic bouton (A) and an axonal terminal (B). MT-based long-distance mitochondrial transport relies on MT polarity. In axons, the MT’s plus ends (+) are oriented toward axonal terminals whereas minus ends (−) are directed toward the soma. Thus, KIF5 motors are responsible for anterograde transport to distal synaptic terminals whereas dynein motors return mitochondria to the soma. The motor adaptor Trak proteins can mediate both KIF5- and dynein-driven bi-directional transport of axonal mitochondria (van Spronsen et al., 2013). Myosin motors likely drive short-range mitochondrial movement at presynaptic terminals where enriched actin filaments constitute cytoskeletal architecture. Motile mitochondria can be recruited into stationary pools via dynamic anchoring interactions between syntaphilin and MTs (Kang et al., 2008) or via an unidentified actin-based anchoring receptor (Chada and Hollenbeck, 2004). Such anchoring mechanisms ensure neuronal mitochondria are adequately distributed along axons and at synapses, where constant energy supply is crucial (figure courtesy of Qian Cai, Department of Cell Biology and Neuroscience, Rutgers University, Piscataway, NJ).
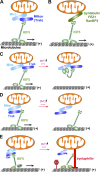
Figure 2.
Activity-dependent regulation of mitochondrial transport. (A) The Miro–Milton (or Miro–Trak) adaptor complex mediates KIF5-driven mitochondrial transport. (B) Syntabulin, FEZ1, and RanBP2 serve as an alternative KIF5 motor adaptor in driving mitochondrial anterograde transport. (C and D) Miro-Ca2+ models in regulating mitochondrial motility. Miro contains Ca2+-binding EF-hand motifs. The C-terminal cargo-binding domain of KIF5 motors binds to the Miro–Trak adaptor complex. (C) Ca2+ binding to Miro’s EF-hands induces the motor domain to disconnect with MTs and thus prevents motor–MT engagement (Wang and Schwarz, 2009). (D) Alternatively, Ca2+ binding releases KIF5 motors from mitochondria (MacAskill et al., 2009a). Thus, Ca2+ influx after synaptic activity arrests motile mitochondria at activated synapses. (E) Syntaphilin-mediated “engine-switch and brake” model. A Miro-Ca2+–sensing pathway triggers the binding switch of KIF5 motors from the Miro–Trak adaptor complex to anchoring protein syntaphilin, which immobilizes axonal mitochondria via inhibiting motor ATPase activity. Thus, syntaphilin turns off the “KIF5 motor engine” by sensing a “stop sign” (elevated Ca2+) and putting a brake on mitochondria, thereby anchoring them in place on MTs. When in their stationary status, KIF5 motor–loaded mitochondria remain associated with the MT tracks while KIF5 ATPase is in an inactive state (Chen and Sheng, 2013; Figure courtesy of Qian Cai).
Figure 3.
Motile mitochondria passing by synapses contribute to presynaptic variation. A stationary mitochondrion within presynaptic terminals powers neurotransmission by stable and continuous ATP supply (left). Conversely, in the absence of a mitochondrion within a presynaptic bouton (right), there is no stable on-site ATP supply; a motile mitochondrion passing through this bouton temporally supplies ATP, thus changing synaptic energy levels and influencing ATP-dependent synaptic functions over time when mitochondrial distribution and motility are altered. Therefore, the fluctuation of synaptic ATP levels resulting from mitochondrial movement is one of the primary sources for the wide variability of synaptic vesicle release and amplitudes of excitatory postsynaptic currents (EPSCs; Sun et al., 2013).
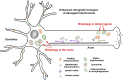
Figure 4.
Functional interplay between mitochondrial transport and mitophagy. Chronically dissipating mitochondrial membrane potential (Δψm) by prolonged treatment of low concentrations of Δψm-uncoupling reagents accumulates Parkin-targeted mitochondria in the soma and proximal regions. Such compartmental restriction is a result of altered motility of depolarized mitochondria with reduced anterograde and relatively enhanced retrograde transport, thus reducing anterograde flux of damaged mitochondria into distal processes (Cai et al., 2012). This spatial process allows neurons to efficiently remove dysfunctional mitochondria from distal axons via the autophagy–lysosomal pathway in the soma, where mature lysosomes are relatively enriched. Damaged mitochondria at axonal terminals can also recruit Parkin for mitophagy once they are anchored by syntaphilin (Cai et al., 2012) or immobilized by turnover of the motor adaptor Miro on the mitochondrial surface (Weihofen et al., 2009; Chan et al., 2011; Wang et al., 2011; Yoshii et al., 2011; Liu et al., 2012; Sarraf et al., 2013). Autophagosomes including those engulfing damaged mitochondria at axonal terminals transport predominantly to the soma for maturation and more efficient degradation of cargoes within acidic lysosomes (Maday et al., 2012).
Similar articles
- Regulation of mitochondrial transport in neurons.
Lin MY, Sheng ZH. Lin MY, et al. Exp Cell Res. 2015 May 15;334(1):35-44. doi: 10.1016/j.yexcr.2015.01.004. Epub 2015 Jan 19. Exp Cell Res. 2015. PMID: 25612908 Free PMC article. Review. - Regulation of axonal mitochondrial transport and its impact on synaptic transmission.
Cai Q, Davis ML, Sheng ZH. Cai Q, et al. Neurosci Res. 2011 May;70(1):9-15. doi: 10.1016/j.neures.2011.02.005. Epub 2011 Feb 23. Neurosci Res. 2011. PMID: 21352858 Free PMC article. Review. - Developmental regulation of microtubule-based trafficking and anchoring of axonal mitochondria in health and diseases.
Cheng XT, Sheng ZH. Cheng XT, et al. Dev Neurobiol. 2021 Apr;81(3):284-299. doi: 10.1002/dneu.22748. Epub 2020 May 2. Dev Neurobiol. 2021. PMID: 32302463 Free PMC article. Review. - The Interplay of Axonal Energy Homeostasis and Mitochondrial Trafficking and Anchoring.
Sheng ZH. Sheng ZH. Trends Cell Biol. 2017 Jun;27(6):403-416. doi: 10.1016/j.tcb.2017.01.005. Epub 2017 Feb 20. Trends Cell Biol. 2017. PMID: 28228333 Free PMC article. Review. - Mitochondrial transport and docking in axons.
Cai Q, Sheng ZH. Cai Q, et al. Exp Neurol. 2009 Aug;218(2):257-67. doi: 10.1016/j.expneurol.2009.03.024. Epub 2009 Mar 31. Exp Neurol. 2009. PMID: 19341731 Free PMC article. Review.
Cited by
- Is SIRT3 and Mitochondria a Reliable Target for Parkinson's Disease and Aging? A Narrative Review.
Kandy AT, Chand J, Baba MZ, Subramanian G. Kandy AT, et al. Mol Neurobiol. 2024 Sep 17. doi: 10.1007/s12035-024-04486-w. Online ahead of print. Mol Neurobiol. 2024. PMID: 39287746 Review. - An aggregated mitochondrial distribution in preimplantation embryos disrupts nuclear morphology, function, and developmental potential.
Lee IW, Tazehkand AP, Sha ZY, Adhikari D, Carroll J. Lee IW, et al. Proc Natl Acad Sci U S A. 2024 Jul 2;121(27):e2317316121. doi: 10.1073/pnas.2317316121. Epub 2024 Jun 25. Proc Natl Acad Sci U S A. 2024. PMID: 38917013 Free PMC article. - Novel Insights into Psychosis and Antipsychotic Interventions: From Managing Symptoms to Improving Outcomes.
Sfera A, Imran H, Sfera DO, Anton JJ, Kozlakidis Z, Hazan S. Sfera A, et al. Int J Mol Sci. 2024 May 28;25(11):5904. doi: 10.3390/ijms25115904. Int J Mol Sci. 2024. PMID: 38892092 Free PMC article. Review. - Activity-dependent mitochondrial ROS signaling regulates recruitment of glutamate receptors to synapses.
Doser RL, Knight KM, Deihl EW, Hoerndli FJ. Doser RL, et al. Elife. 2024 Mar 14;13:e92376. doi: 10.7554/eLife.92376. Elife. 2024. PMID: 38483244 Free PMC article. - Mitochondria: A Promising Convergent Target for the Treatment of Amyotrophic Lateral Sclerosis.
Cunha-Oliveira T, Montezinho L, Simões RF, Carvalho M, Ferreiro E, Silva FSG. Cunha-Oliveira T, et al. Cells. 2024 Jan 29;13(3):248. doi: 10.3390/cells13030248. Cells. 2024. PMID: 38334639 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous