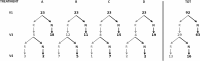Rifaximin modulates the vaginal microbiome and metabolome in women affected by bacterial vaginosis - PubMed (original) (raw)
Rifaximin modulates the vaginal microbiome and metabolome in women affected by bacterial vaginosis
Luca Laghi et al. Antimicrob Agents Chemother. 2014 Jun.
Abstract
Bacterial vaginosis (BV) is a common vaginal disorder characterized by the decrease of lactobacilli and overgrowth of Gardnerella vaginalis and resident anaerobic vaginal bacteria. In the present work, the effects of rifaximin vaginal tablets on vaginal microbiota and metabolome of women affected by BV were investigated by combining quantitative PCR and a metabolomic approach based on (1)H nuclear magnetic resonance. To highlight the general trends of the bacterial communities and metabolomic profiles in response to the antibiotic/placebo therapy, a multivariate statistical strategy was set up based on the trajectories traced by vaginal samples in a principal component analysis space. Our data demonstrated the efficacy of rifaximin in restoring a health-like condition in terms of both bacterial communities and metabolomic features. In particular, rifaximin treatment was significantly associated with an increase in the lactobacillus/BV-related bacteria ratio, as well as with an increase in lactic acid concentration and a decrease of a pool of metabolites typically produced by BV-related bacteria (acetic acid, succinate, short-chain fatty acids, and biogenic amines). Among the tested dosages of rifaximin (100 and 25 mg for 5 days and 100 mg for 2 days), 25 mg for 5 days was found to be the most effective.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
FIG 1
Remission (R) or nonremission (N) evaluated at the follow-up visits V3 and V4 for the treatment groups A (100 mg/day for 5 days), B (25 mg/day for 5 days), C (100 mg/day for 2 days), and D (placebo for 5 days) and for the totality of the women (TOT).
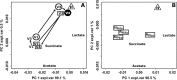
FIG 2
(A) Biplot of a PCA performed on the autoscaled qPCR data related to BV-affected women at visits V1 and V3 and healthy control women. The squares and circles represent BV-affected women at V1 and V3, respectively, with the open and filled symbols indicating women in remission (R) or not in remission (N), respectively, after rifaximin or placebo treatment. The gray triangles represent healthy women (H). Median values of the sample groups are indicated as V1, R-V3, N-V3, and H. (B) Biplot of a PCA performed on qPCR data related to BV-affected women at visit V1 and to healthy women. R and N indicate the median values of women grouped according to the response to rifaximin (RRifa [n = 25] and NRifa [n = 44], respectively) or placebo (RPbo [n = 4] and NPbo [n = 19], respectively) at V3, while H indicates the median values of healthy women (H; n = 10). Both trajectories (V1→R-V3 and V1→N-V3) indicate significant differences. Abbreviations: Lact, Lactobacillus; Gard, G. vaginalis; Atop, Atopobium; Prev, Prevotella; Veil, Veillonella; Mhom, M. hominis; Mobi, Mobiluncus; expl.var, explained variance.
FIG 3
Biplot of a PCA calculated on the autoscaled qPCR data related to healthy women (triangle) and BV-affected women in remission at both V3 and V4 (circles), in remission at V3 but not at V4 (squares), and not in remission at V3 (no symbol). To limit the sample superimposition, data are represented separately for women subjected to placebo (A) and rifaximin (B) treatment. Median values of the samples collected from healthy women (H) and BV-affected women at V1, V3, and V4 are shown. The number of women (n) for each sample group is reported in Fig. 1. Continuous lines indicate significant differences between V1–V3 and V3–V4 trajectories, while dashed lines indicate nonsignificant differences. Full V4 symbols indicate significant differences between V1 and V4. expl.var, explained variance.
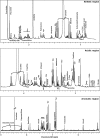
FIG 4
1H-NMR spectrum of a vaginal fluid sample collected from a BV-affected woman at the baseline.
FIG 5
(A) Biplot of a PCA calculated on the entire set of NMR spectra. Median values of the samples collected from healthy women (triangle) and BV-affected women in remission at both V3 and V4 (circles), in remission at V3 but not at V4 (squares), and not in remission at V3 (no symbols) are shown. (B) Biplot of a PCA calculated on NMR data related to BV-affected women at visit V1 and to healthy women. R and N indicate the median values of women grouped according to the response to rifaximin (RRifa, n = 25; NRifa, n = 44) or placebo (RPbo, n = 4; NPbo, n = 19) at V3, while H indicates the median values of healthy women (H; n = 10). Continuous lines in panel A indicate significant differences between V1–V3 and V3–V4 trajectories, while dashed lines indicate nonsignificant differences. Full V4 symbols indicate significant differences between V1 and V4. expl.var, explained variance.
FIG 6
Biplot of a PCA calculated on the autoscaled concentrations of the molecules associated with the presence of BV-related bacteria measured in healthy women (triangle) and BV-affected women in remission at both V3 and V4 (circles), in remission at V3 but not at V4 (squares), and not in remission at V3 (no symbol). To limit the sample superimposition, data are represented separately for women subjected to placebo (A) or rifaximin (B) treatment. Median values of the samples collected from healthy women (H) and BV-affected women at V1, V3, and V4 are shown. The number of women (n) for each sample group is reported in Fig. 1. Continuous lines indicate significant differences between V1–V3 and V3–V4 trajectories, while dashed lines indicate nonsignificant differences. Full V4 symbols indicate significant differences between V1 and V4. expl.var, explained variance.
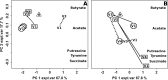
FIG 7
Biplots of PCA models built on the autoscaled differences for V3–V1 between qPCR (A) and metabolic (B) data. The medians of the samples from women in remission or not in remission at V3 are indicated as R and N, respectively, while rifaximin dosing regimens are indicated as subscripts. Abbreviations: Lact, Lactobacillus; Gard, G. vaginalis; Atop, Atopobium; Prev, Prevotella; Veil, Veillonella; Mhom, M. hominis; Mobi, Mobiluncus; expl.var, explained variance.
Similar articles
- Efficacy of rifaximin vaginal tablets in treatment of bacterial vaginosis: a molecular characterization of the vaginal microbiota.
Cruciani F, Brigidi P, Calanni F, Lauro V, Tacchi R, Donders G, Peters K, Guaschino S, Vitali B. Cruciani F, et al. Antimicrob Agents Chemother. 2012 Aug;56(8):4062-70. doi: 10.1128/AAC.00061-12. Epub 2012 May 14. Antimicrob Agents Chemother. 2012. PMID: 22585228 Free PMC article. Clinical Trial. - Development of a microarray-based tool to characterize vaginal bacterial fluctuations and application to a novel antibiotic treatment for bacterial vaginosis.
Cruciani F, Biagi E, Severgnini M, Consolandi C, Calanni F, Donders G, Brigidi P, Vitali B. Cruciani F, et al. Antimicrob Agents Chemother. 2015 May;59(5):2825-34. doi: 10.1128/AAC.00225-15. Epub 2015 Mar 2. Antimicrob Agents Chemother. 2015. PMID: 25733514 Free PMC article. - Effectiveness of the two microorganisms Lactobacillus fermentum LF15 and Lactobacillus plantarum LP01, formulated in slow-release vaginal tablets, in women affected by bacterial vaginosis: a pilot study.
Vicariotto F, Mogna L, Del Piano M. Vicariotto F, et al. J Clin Gastroenterol. 2014 Nov-Dec;48 Suppl 1:S106-12. doi: 10.1097/MCG.0000000000000226. J Clin Gastroenterol. 2014. PMID: 25291116 Clinical Trial. - Probiotics for the treatment of women with bacterial vaginosis.
Falagas M, Betsi GI, Athanasiou S. Falagas M, et al. Clin Microbiol Infect. 2007 Jul;13(7):657-64. doi: 10.1111/j.1469-0691.2007.01688.x. Clin Microbiol Infect. 2007. PMID: 17633390 Review. - Vaginal microbiome.
Buchta V. Buchta V. Ceska Gynekol. 2018 Winter;83(5):371-379. Ceska Gynekol. 2018. PMID: 30848142 Review. English.
Cited by
- Insights Into Vaginal Bacterial Communities and Metabolic Profiles of Chlamydia trachomatis Infection: Positioning Between Eubiosis and Dysbiosis.
Parolin C, Foschi C, Laghi L, Zhu C, Banzola N, Gaspari V, D'Antuono A, Giordani B, Severgnini M, Consolandi C, Salvo M, Cevenini R, Vitali B, Marangoni A. Parolin C, et al. Front Microbiol. 2018 Mar 28;9:600. doi: 10.3389/fmicb.2018.00600. eCollection 2018. Front Microbiol. 2018. PMID: 29643849 Free PMC article. - The genital tract and rectal microbiomes: their role in HIV susceptibility and prevention in women.
Abdool Karim SS, Baxter C, Passmore JS, McKinnon LR, Williams BL. Abdool Karim SS, et al. J Int AIDS Soc. 2019 May;22(5):e25300. doi: 10.1002/jia2.25300. J Int AIDS Soc. 2019. PMID: 31144462 Free PMC article. Review. - Vaginal microbiome and metabolome highlight specific signatures of bacterial vaginosis.
Vitali B, Cruciani F, Picone G, Parolin C, Donders G, Laghi L. Vitali B, et al. Eur J Clin Microbiol Infect Dis. 2015 Dec;34(12):2367-76. doi: 10.1007/s10096-015-2490-y. Epub 2015 Sep 18. Eur J Clin Microbiol Infect Dis. 2015. PMID: 26385347 - The Complex Link between the Female Genital Microbiota, Genital Infections, and Inflammation.
Dabee S, Passmore JS, Heffron R, Jaspan HB. Dabee S, et al. Infect Immun. 2021 Apr 16;89(5):e00487-20. doi: 10.1128/IAI.00487-20. Print 2021 Apr 16. Infect Immun. 2021. PMID: 33558324 Free PMC article. Review. - Bacterial Vaginosis: Current Diagnostic Avenues and Future Opportunities.
Redelinghuys MJ, Geldenhuys J, Jung H, Kock MM. Redelinghuys MJ, et al. Front Cell Infect Microbiol. 2020 Aug 11;10:354. doi: 10.3389/fcimb.2020.00354. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32850469 Free PMC article. Review.
References
- Workowski KA, Berman SM. 2010. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm. Rep. 59:1–110 - PubMed
- Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, Horvath LB, Kuzevska I, Fairley CK. 2006. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 193:1478–1486. 10.1086/503780 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical