MiRNA 17 family regulates cisplatin-resistant and metastasis by targeting TGFbetaR2 in NSCLC - PubMed (original) (raw)
MiRNA 17 family regulates cisplatin-resistant and metastasis by targeting TGFbetaR2 in NSCLC
Zeyong Jiang et al. PLoS One. 2014.
Expression of concern in
- Expression of Concern: miRNA 17 Family Regulates Cisplatin-Resistant and Metastasis by Targeting TGFbetaR2 in NSCLC.
PLOS ONE Editors. PLOS ONE Editors. PLoS One. 2019 Sep 19;14(9):e0222896. doi: 10.1371/journal.pone.0222896. eCollection 2019. PLoS One. 2019. PMID: 31536592 Free PMC article. No abstract available.
Abstract
MicroRNAs (miRNAs) have been proven to play crucial roles in cancer, including tumor chemotherapy resistance and metastasis of non-small-cell lung cancer (NSCLC). TGFβ signal pathway abnormality is widely found in cancer and correlates with tumor proliferation, apoptosis and metastasis. Here, miR-17, 20a, 20b were detected down-regulated in A549/DDP cells (cisplatin resistance) compared with A549 cells (cisplatin sensitive). Over-expression of miR-17, 20a, 20b can not only decrease cisplatin-resistant but also reduce migration by inhibiting epithelial-to-mesenchymal transition (EMT) in A549/DDP cells. These functions of miR-17, 20a, 20b may be caused at least in part via inhibition of TGFβ signal pathway, as miR-17, 20a, 20b are shown to directly target and repress TGF-beta receptor 2 (TGFβR2) which is an important component of TGFβ signal pathway. Consequently, our study suggests that miRNA 17 family (including miR-17, 20a, 20b) can act as TGFβR2 suppressor for reversing cisplatin-resistant and suppressing metastasis in NSCLC.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
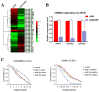
Figure 1. miR-17, 20a, 20b are low-expressed in A549/DDP cells and regulate cisplatin-resistant.
(A) Clustering of cisplatin-resistant related miRNAs in A549 and A549/DDP cells. The vertical axis corresponds to the expression difference of miRNAs, A549 means A549 cells compared with A549/DDP cells in one microarray assay, A549/DDP means A549/DDP cells compared with A549 cells in another repeat microarray assay. The horizontal axis corresponds to miRNAs. miRNA expression levels were depicted as color variation from red (high expression) to green (low expression) according to color bar scale. (B) RT-PCR validated that miR-17, 20a, 20b expression levels were significantly lower in A549/DDP cells. (C) Over-expression or inhibition of miR-17, 20a, 20b significantly increased or decreased the growth-inhibitory effect of cisplatin in A549/DDP cells or A549 cells. *P<0.05. Results were representative of three experiments.
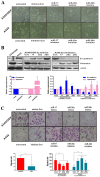
Figure 2. miR-17, 20a, 20b regulate EMT and migration.
(A) Morphology of A549 and A549/DDP cells with or without miRNAs treatment. (B) mRNA and protein expression levels of E-cadherin and Vimentin in A549 and A549/DDP cells with or without miRNAs treatment were measured by RT-PCR and Western Blotting. (C) Transfer cells in migration assays were detected by transwell-chamber culture systems. Bar graphs show the number of migratory cells. *P<0.05. Results were representative of three experiments.
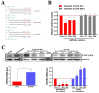
Figure 3. miR-17, 20a, 20b target TGFβR2 by directly binding to the TGFβR2 mRNA 3′-UTR.
(A) Site-directed mutagenesis targeting potential miR-17, 20a, 20b binding sites (MUT) on the TGFβR2 mRNA 3′-UTR-luciferase construct. (B) Luciferase activities significantly decreased in the WT TGFβR2 mRNA 3′-UTR-luciferase plasmid transfected A549 cells after transfection of miR-17, 20a, 20b mimics. Effect was blocked in the MUT plasmid transfected A549 cells. (C) By RT-PCR and Western Blotting, the expression of TGFβR2 increased significantly in A549/DDP cells compared with A549 cells. The expression of TGFβR2 decreased in A549/DDP cells after transfection of miR-17, 20a, 20b mimics and increased in A549 cells after transfection of miR-17, 20a, 20b inhibitors. *P<0.05. Results were representative of three experiments.
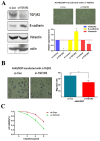
Figure 4. TGFβR2 silenced restrain cisplatin-resistant and reduce migration in A549/DDP cells.
(A) After inhibition of TGFβR2 in A549/DDP cells by transfected with si-TGFβR2, the EMT phenotype changed evidenced by over-expression of E-cadherin and down-regulation of Vimentin, and (B) the migratory capability also significantly decreased. Bar graphs show the number of migratory cells. (C) TGFβR2 silenced induced by siRNA led to decrease cisplatin resistance, as shown by a significantly growth inhibition curve of cisplatin in A549/DDP cells. *P<0.05. Results were representative of three experiments.
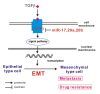
Figure 5. miR-17 family regulate cisplatin-resistant and metastasis by targeting TGFβR2 in NSCLC.
TGFβ signal pathway can induce epithelial cells into mesenchymal cells by EMT, and mesenchymal cells show the potential of drug resistance and metastasis. In this study, miR-17, 20a, 20b can inhibit the TGFβ signal pathway by targeting TGFβR2, consequently, inhibition of miR-17, 20a, 20b can induce EMT and lead to cisplatin-resistant and migration in A549/DDP cells.
Similar articles
- MiR-181b regulates cisplatin chemosensitivity and metastasis by targeting TGFβR1/Smad signaling pathway in NSCLC.
Wang X, Chen X, Meng Q, Jing H, Lu H, Yang Y, Cai L, Zhao Y. Wang X, et al. Sci Rep. 2015 Dec 1;5:17618. doi: 10.1038/srep17618. Sci Rep. 2015. PMID: 26620926 Free PMC article. - The miR 495-UBE2C-ABCG2/ERCC1 axis reverses cisplatin resistance by downregulating drug resistance genes in cisplatin-resistant non-small cell lung cancer cells.
Guo J, Jin D, Wu Y, Yang L, Du J, Gong K, Chen W, Dai J, Miao S, Xi S. Guo J, et al. EBioMedicine. 2018 Sep;35:204-221. doi: 10.1016/j.ebiom.2018.08.001. Epub 2018 Aug 23. EBioMedicine. 2018. PMID: 30146342 Free PMC article. Retracted. - MiR-124 changes the sensitivity of lung cancer cells to cisplatin through targeting STAT3.
Qi MM, Ge F, Chen XJ, Tang C, Ma J. Qi MM, et al. Eur Rev Med Pharmacol Sci. 2019 Jun;23(12):5242-5250. doi: 10.26355/eurrev_201906_18190. Eur Rev Med Pharmacol Sci. 2019. PMID: 31298375 - [Advances in the relationship between microRNA and cisplatin resistance of lung cancer].
Zhang F, Li Y, Zhou Q. Zhang F, et al. Zhongguo Fei Ai Za Zhi. 2014 Mar;17(3):269-72. doi: 10.3779/j.issn.1009-3419.2014.03.15. Zhongguo Fei Ai Za Zhi. 2014. PMID: 24667267 Free PMC article. Review. Chinese. - Role of microRNAs in metastasis of non-small cell lung cancer.
Li S, Gao M, Li Z, Song L, Gao X, Han J, Wang F, Chen Y, Li W, Yang J, Han X. Li S, et al. Front Biosci (Landmark Ed). 2016 Mar 1;21(5):998-1005. doi: 10.2741/4436. Front Biosci (Landmark Ed). 2016. PMID: 26709755 Review.
Cited by
- Composition of Conditioned Media from Radioresistant and Chemoresistant Cancer Cells Reveals miRNA and Other Secretory Factors Implicated in the Development of Resistance.
Molodtsova D, Guryev DV, Osipov AN. Molodtsova D, et al. Int J Mol Sci. 2023 Nov 19;24(22):16498. doi: 10.3390/ijms242216498. Int J Mol Sci. 2023. PMID: 38003688 Free PMC article. Review. - The Roles of MicroRNA in Lung Cancer.
Wu KL, Tsai YM, Lien CT, Kuo PL, Hung AJ. Wu KL, et al. Int J Mol Sci. 2019 Mar 31;20(7):1611. doi: 10.3390/ijms20071611. Int J Mol Sci. 2019. PMID: 30935143 Free PMC article. Review. - miR-1269b Drives Cisplatin Resistance of Human Non-Small Cell Lung Cancer via Modulating the PTEN/PI3K/AKT Signaling Pathway.
Yang W, Xiao W, Cai Z, Jin S, Li T. Yang W, et al. Onco Targets Ther. 2020 Jan 7;13:109-118. doi: 10.2147/OTT.S225010. eCollection 2020. Onco Targets Ther. 2020. PMID: 32021259 Free PMC article. - Expression of Concern: miRNA 17 Family Regulates Cisplatin-Resistant and Metastasis by Targeting TGFbetaR2 in NSCLC.
PLOS ONE Editors. PLOS ONE Editors. PLoS One. 2019 Sep 19;14(9):e0222896. doi: 10.1371/journal.pone.0222896. eCollection 2019. PLoS One. 2019. PMID: 31536592 Free PMC article. No abstract available. - MicroRNAs associated with therapy of non-small cell lung cancer.
Lu J, Zhan Y, Feng J, Luo J, Fan S. Lu J, et al. Int J Biol Sci. 2018 Mar 10;14(4):390-397. doi: 10.7150/ijbs.22243. eCollection 2018. Int J Biol Sci. 2018. PMID: 29725260 Free PMC article. Review.
References
- Mallini P, Lennard T, Kirby J, Meeson A (2014) Epithelial-to-mesenchymal transition: What is the impact on breast cancer stem cells and drug resistance. Cancer Treat Rev 40: 341–348. - PubMed
- Tan J, You Y, Xu T, Yu P, Wu D, et al. (2013) Par-4 downregulation confers cisplatin resistance in pancreatic cancer cells via PI3K/Akt pathway-dependent EMT. Toxicol Lett 224: 7–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical