Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells - PubMed (original) (raw)
. 2014 Jun;13(6):599-604.
doi: 10.1038/nmat3945. Epub 2014 Apr 13.
Affiliations
- PMID: 24728461
- PMCID: PMC4051885
- DOI: 10.1038/nmat3945
Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells
Yubing Sun et al. Nat Mater. 2014 Jun.
Abstract
Our understanding of the intrinsic mechanosensitive properties of human pluripotent stem cells (hPSCs), in particular the effects that the physical microenvironment has on their differentiation, remains elusive. Here, we show that neural induction and caudalization of hPSCs can be accelerated by using a synthetic microengineered substrate system consisting of poly(dimethylsiloxane) micropost arrays (PMAs) with tunable mechanical rigidities. The purity and yield of functional motor neurons derived from hPSCs within 23 days of culture using soft PMAs were improved more than fourfold and tenfold, respectively, compared with coverslips or rigid PMAs. Mechanistic studies revealed a multi-targeted mechanotransductive process involving Smad phosphorylation and nucleocytoplasmic shuttling, regulated by rigidity-dependent Hippo/YAP activities and actomyosin cytoskeleton integrity and contractility. Our findings suggest that substrate rigidity is an important biophysical cue influencing neural induction and subtype specification, and that microengineered substrates can thus serve as a promising platform for large-scale culture of hPSCs.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures
Figure 1
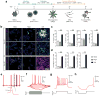
Soft substrates promote neuroepithelial conversion while inhibiting neural crest differentiation of hESCs in a BMP4-dependent manner. (a) Schematic diagram showing experimental design of hESC neural induction. hESCs were cultured for 8 d in neural induction medium containing the dual Smad inhibitors, SB 431542 (SB, 10 μM) and LDN 193189 (LDN, 100 nM). (b) Representative immunofluorescence images showing Pax6+ NEs and AP2+ NCs after 8 d of culture with (top; 100 nM) or without (bottom) LDN on vitronectin-coated coverslips and rigid (E = 1,200 kPa) and soft (E = 5 kPa) PMAs. Scale bar, 100 μm. (c) Bar graph showing percentage of Pax6+ NEs on coverslips and rigid and soft PMAs at day 4, 6, and 8. (d) Western blotting showing Pax6 and Sox1 expression levels in hESCs cultured for 8 d on coverslips and rigid and soft PMAs. (e) Bar plot showing percentages of Pax6+ NEs and AP2+ NCs at day 8 as a function of substrate rigidity and LDN concentration. Data represents the mean ± s.e.m with n = 3. _P_-values were calculated using one-way ANOVA, followed by Tukey post hoc analysis. *, P < 0.05; **, P < 0.01.
Figure 2
Purity and yield of functional motor neurons (MNs) derived from hESCs are improved on soft substrates. (a) Schematic diagram showing experimental design for sequential neural induction, patterning, and functional maturation of MNs from hESCs. hESCs were cultured on vitronectin-coated coverslips and rigid (E = 1,200 kPa) and soft (E = 5 kPa) PMAs in neural induction medium containing the dual Smad inhibitors SB and LDN for 8 d and then in MN differentiation medium containing purmorphamine (Pur), basic fibroblast growth factor (bFGF), and retinoic acid (RA) for an additional 8 d. Putative MN progenitor cells collected at day 16 were transferred onto coverslips and cultured in MN maturation medium containing brain-derived neurotrophic factor (BDNF), ascorbic acid, cyclic adenosine monophosphate (cAMP), and insulin-like growth factor 1 (IGF-1) for another 14 d. (b) Representative immunofluorescence images showing Tuj1+, Isl1+, and HB9+ cells at day 23 (top), and ChAT+ cells at day 30 (bottom). Scale bar, 100 μm. (c&d) Bar plots showing percentages (c) and relative numbers (d) of Tuj1+, Isl1+, and HB9+ cells at day 23 as a function of substrate rigidity. Data in d was normalized to values from coverslips. Data represents the mean ± s.e.m with n ≥3. _P_-values were calculated using two-side unpaired student _t_-tests. *: P < 0.05; **: P < 0.01. (e–h) Electrophysiological characterization of functional MNs derived from soft PMAs at day 30 using whole-cell patch clamp. e: spontaneous action potential (AP) with resting membrane potential of −64.5 mV; f: voltage response to current step injection; g: instantaneous frequency change or spike frequency adaptation (SFA) evoked with positive current injection; h: post-inhibitory rebound (PIR) after hyperpolarizing current injection.
Figure 3
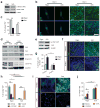
Soft substrates promote hESC neuroepithelial conversion through a multi-targeted mechanotransductive process involving mechanosensitive Smad phosphorylation and nucleocytoplasmic shuttling regulated by rigidity-dependent Hippo-YAP activities and the actomyosin cytoskeleton (CSK). (a) Western blotting of total and phosphorylated Smad 1/5/8 (p-Smad 1/5/8) in hESCs cultured in neural induction medium for 3 d on rigid (E = 1,200 kPa) and soft (E = 5 kPa) PMAs. (b&c) Representative immunofluorescence images (b) and bar plot (c) showing rigidity-dependent subcellular localization of YAP in hESCs at day 0 and day 3. Scale bar in b, 50 μm. (d) Western blotting for YAP, TAZ, Smad 1/5/8, and Smad 2/3 in nuclear and cytoplasmic protein fractions from hESCs cultured for 3 d on rigid and soft PMAs. (e) Western blotting for phosphorylated YAP S127 (p-YAP S127) and YAP in whole cell lysates of hESCs after 3 d of culture on rigid and soft PMAs. (f&g) Representative immunofluorescence images (f) and bar plot (g) showing the percentage of Pax6+ NEs derived from scramble control and siLats1 knockdown hESCs after 6 d of culture on rigid and soft PMAs. Scale bar in f, 50 μm. (h) Bar graph showing the percentage of hESCs with nuclear localization of YAP after 3 d of culture with different drug supplementations, as indicated. (i&j) Representative immunofluorescence images (i) and bar plot (j) showing the percentage of Pax6+ NEs derived from hESCs on rigid and soft PMAs after 6 d of culture with different drug supplementations, as indicated. Scale bar in i, 50 μm. In Western blots β-actin and GAPDH were used as protein loading controls and lamin A/C for nuclear fraction control. Data represents the mean ± s.e.m with n ≥ 3. _P_-values in c & g and h & j were calculated using two-side unpaired student _t_-tests and one way ANOVA followed by Tukey post hoc analysis, respectively. *, P < 0.05; **, P < 0.01.
Comment in
- Stem cell differentiation: yielding substrates for neurons.
Lowry ER, Henderson CE. Lowry ER, et al. Nat Mater. 2014 Jun;13(6):543-4. doi: 10.1038/nmat3992. Nat Mater. 2014. PMID: 24845991 No abstract available. - Stem cells go soft: pliant substrate surfaces enhance motor neuron differentiation.
Wang N. Wang N. Cell Stem Cell. 2014 Jun 5;14(6):701-3. doi: 10.1016/j.stem.2014.05.007. Cell Stem Cell. 2014. PMID: 24905160 Free PMC article.
Similar articles
- Stem cells go soft: pliant substrate surfaces enhance motor neuron differentiation.
Wang N. Wang N. Cell Stem Cell. 2014 Jun 5;14(6):701-3. doi: 10.1016/j.stem.2014.05.007. Cell Stem Cell. 2014. PMID: 24905160 Free PMC article. - Stem cell differentiation: yielding substrates for neurons.
Lowry ER, Henderson CE. Lowry ER, et al. Nat Mater. 2014 Jun;13(6):543-4. doi: 10.1038/nmat3992. Nat Mater. 2014. PMID: 24845991 No abstract available. - Nanotopography regulates motor neuron differentiation of human pluripotent stem cells.
Chen W , Han S , Qian W , Weng S , Yang H , Sun Y , Villa-Diaz LG , Krebsbach PH , Fu J . Chen W , et al. Nanoscale. 2018 Feb 15;10(7):3556-3565. doi: 10.1039/c7nr05430k. Nanoscale. 2018. PMID: 29410983 Free PMC article. - Regulation of YAP/TAZ Activity by Mechanical Cues: An Experimental Overview.
Dupont S. Dupont S. Methods Mol Biol. 2019;1893:183-202. doi: 10.1007/978-1-4939-8910-2_15. Methods Mol Biol. 2019. PMID: 30565135 Review. - Yes-Associated Protein and PDZ Binding Motif: A Critical Signaling Pathway in the Control of Human Pluripotent Stem Cells Self-Renewal and Differentiation.
Shi J, Farzaneh M, Khoshnam SE. Shi J, et al. Cell Reprogram. 2020 Apr;22(2):55-61. doi: 10.1089/cell.2019.0084. Epub 2020 Mar 3. Cell Reprogram. 2020. PMID: 32125897 Review.
Cited by
- Role of YAP/TAZ in Cell Lineage Fate Determination and Related Signaling Pathways.
Heng BC, Zhang X, Aubel D, Bai Y, Li X, Wei Y, Fussenegger M, Deng X. Heng BC, et al. Front Cell Dev Biol. 2020 Jul 30;8:735. doi: 10.3389/fcell.2020.00735. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850847 Free PMC article. Review. - Biomaterials and Advanced Biofabrication Techniques in hiPSCs Based Neuromyopathic Disease Modeling.
Sun J, Ma X, Chu HT, Feng B, Tuan RS, Jiang Y. Sun J, et al. Front Bioeng Biotechnol. 2019 Nov 29;7:373. doi: 10.3389/fbioe.2019.00373. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31850331 Free PMC article. Review. - Tissue mechanics, an important regulator of development and disease.
Ayad NME, Kaushik S, Weaver VM. Ayad NME, et al. Philos Trans R Soc Lond B Biol Sci. 2019 Aug 19;374(1779):20180215. doi: 10.1098/rstb.2018.0215. Epub 2019 Jul 1. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31431174 Free PMC article. Review. - Biomaterials and engineered microenvironments to control YAP/TAZ-dependent cell behaviour.
Brusatin G, Panciera T, Gandin A, Citron A, Piccolo S. Brusatin G, et al. Nat Mater. 2018 Dec;17(12):1063-1075. doi: 10.1038/s41563-018-0180-8. Epub 2018 Oct 29. Nat Mater. 2018. PMID: 30374202 Free PMC article. Review. - Materials and extracellular matrix rigidity highlighted in tissue damages and diseases: Implication for biomaterials design and therapeutic targets.
Park JH, Jo SB, Lee JH, Lee HH, Knowles JC, Kim HW. Park JH, et al. Bioact Mater. 2022 Jun 16;20:381-403. doi: 10.1016/j.bioactmat.2022.06.003. eCollection 2023 Feb. Bioact Mater. 2022. PMID: 35784640 Free PMC article. Review.
References
- Li XJ, et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01NS062792/NS/NINDS NIH HHS/United States
- 2R01DE016530-06/DE/NIDCR NIH HHS/United States
- R01 DE016530/DE/NIDCR NIH HHS/United States
- R01 AR060837/AR/NIAMS NIH HHS/United States
- R21 HL114011/HL/NHLBI NIH HHS/United States
- R01 NS062792/NS/NINDS NIH HHS/United States
- R01AR060837/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources