Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis - PubMed (original) (raw)
. 2014 Apr 14;25(4):501-15.
doi: 10.1016/j.ccr.2014.03.007.
Miranda Y Fong 2, Yongfen Min 3, George Somlo 4, Liang Liu 5, Melanie R Palomares 6, Yang Yu 5, Amy Chow 2, Sean Timothy Francis O'Connor 2, Andrew R Chin 7, Yun Yen 8, Yafan Wang 9, Eric G Marcusson 10, Peiguo Chu 11, Jun Wu 12, Xiwei Wu 13, Arthur Xuejun Li 14, Zhuo Li 15, Hanlin Gao 16, Xiubao Ren 17, Mark P Boldin 18, Pengnian Charles Lin 3, Shizhen Emily Wang 19
Affiliations
- PMID: 24735924
- PMCID: PMC4016197
- DOI: 10.1016/j.ccr.2014.03.007
Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis
Weiying Zhou et al. Cancer Cell. 2014.
Abstract
Cancer-secreted microRNAs (miRNAs) are emerging mediators of cancer-host crosstalk. Here we show that miR-105, which is characteristically expressed and secreted by metastatic breast cancer cells, is a potent regulator of migration through targeting the tight junction protein ZO-1. In endothelial monolayers, exosome-mediated transfer of cancer-secreted miR-105 efficiently destroys tight junctions and the integrity of these natural barriers against metastasis. Overexpression of miR-105 in nonmetastatic cancer cells induces metastasis and vascular permeability in distant organs, whereas inhibition of miR-105 in highly metastatic tumors alleviates these effects. miR-105 can be detected in the circulation at the premetastatic stage, and its levels in the blood and tumor are associated with ZO-1 expression and metastatic progression in early-stage breast cancer.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
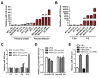
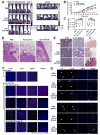
Figure 1. MBC-secreted exosomal RNA regulates migration of endothelial cells
(A) EM images of exosomes secreted by MCF-10A and MDA-MB-231 cells. (B) Primary HMVECs were incubated with DiI-labeled exosomes (red) for 24 hr before fluorescent and phase contrast images were captured. (C) HMVECs incubated with DiI-labeled exosomes for indicated time were analyzed by flow cytometry for DiI uptake. (D) After 48 hr incubation with exosomes or PBS (as control), HMVECs were analyzed for transwell migration and cells that had migrated within 8 hr were quantified from triplicate wells. (E) HMVECs transfected with equal amount of total or small (<200 nt) RNA extracted from MCF-10A or MDA-MB-231 (abbreviated to MDA-231 or 231 in Figures) secreted exosomes, or control RNA (cel-miR-67), were subjected to transwell migration at 48 hr post transfection. *p < 0.005 compared to control group. Results are presented as mean ± SD.
Figure 2. MiR-105 is specifically expressed and secreted by MBC cells and can be transferred to endothelial cells via exosome secretion
Cellular (A) and exosomal (B) RNA were extracted from various breast cell lines and subjected to miR-105 RT-qPCR. Data was normalized to levels of U6 (cellular; A) or miR-16 (exosomal; B), and compared to the non-tumor line MCF-10A. MBC lines originally isolated from pleural effusion are indicated by red columns. (C) RNA was extracted from HMVECs incubated with exosomes of different origins for indicated time and analyzed for miR-105 level using U6 as internal control. At each time point, data was compared to PBS-treated cells. (D) RNA extracted from HMVECs incubated with exosomes of different origins for 24 hr (or PBS as control) was analyzed for the level of pri-miR-105 or pre-miR-105. (E) MDA-MB-231-secreted exosomes were fed to HMVECs in the presence or absence of DRB (20 μM). After 24 hr, RNA extracted from the recipient cells was analyzed for miR-105 level. *p < 0.005 compared to PBS treatment. Results are presented as mean ± SD. (See also Figure S1 and Table S1.)
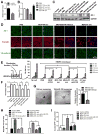
Figure 3. Cancer-secreted miR-105 downregulates tight junctions and destroys the barrier function of endothelial monolayer
(A) HMVECs transduced with miR-105 or vector were analyzed for ZO-1 expression by RT-qPCR. (B) HMVECs treated as indicated were analyzed for the RNA level of ZO-1. (C) HMVECs treated as indicated were analyzed by Western blot. (D) HMVEC monolayers were treated as indicated for 48 hr and analyzed by IF for ZO-1 (green), occludin (red) and VE-cadherin (green). DAPI (blue): cell nuclei. (E) The permeability of treated HMVEC monolayers grown on 0.4-μm filters was measured by the appearance of rhodamine-dextran, which was added to the top well at the beginning of the experiment, in the bottom well during a 1 hr time course. The absorbance at 590 nm at each time point was indicated. Treatment of the HMVEC monolayer with VEGF (50 ng/ml) for 8 hr was included as a positive control to show cytokine-induced permeability. The absorbance at the 1 hr time point was compared to the PBS (control) condition. *p < 0.005. **p > 0.05. (F) HMVEC monolayers grown on filters and treated as indicated were analyzed for TEER. Calculated unit area resistance from triplicate wells was normalized to the control (PBS) treatment. (G) Treatment with miR-105-containing exosomes resulted in a vascular destruction. Vascular sprouting assay was established for 5 days, at which time 1 μg of purified exosomes from MCF10A/vec (control) or MCF10A/miR-105 cells were added into the culture media. Vascular structures were imaged 5 days after the treatment, and representative images were shown (left panel). Vascular sprouts per spheroid were counted and graphed (right panel). At least 50 spheroids were counted in each experiment and the experiment was repeated three times. *p < 0.05. (H) HMVECs treated as indicated were subjected to transwell migration. Cells that had migrated within 8 hr were quantified from triplicate wells. *p < 0.005. (I) HMVEC monolayers grown on 3-μm filters were treated as indicated before GFP-labeled MDA-231-HM cells were seeded in the transwell inserts. After 10 hr, the GFP+ cells on the bottom side of filters were quantified under a fluorescent microscope. *p < 0.005. Results are presented as mean ± SD. (See also Figure S2.)
Figure 4. Cancer-secreted miR-105 induces vascular permeability and promotes metastasis in vivo
(A) Exosomes secreted by MCF10A/vec, MCF10A/miR-105, or MDA-MB-231 cells, or PBS (as control), were intravenously injected into the tail vein of NSG mice (n = 3) twice a week. After 5 injections, tissues were collected for RT-qPCR of miR-105 using U6 as internal control. *p < 0.05. (B) Collected lung and brain tissues were subjected to double-label IF for ZO-1 (green) and CD31 (pink). Structures positive for CD31 are indicated by arrowheads. Bar = 100 μm. (C) In vivo vascular permeability determined by the appearance of intravenously injected rhodamine-dextran (red) (n = 3). Representative images are shown. DAPI (blue): cell nuclei. Bar = 100 μm. (D) Exosomes secreted by MCF-10A or MDA-MB-231 cells, or PBS (as control), were intravenously injected into the tail vein of NSG mice (n = 6) twice a week. After 5 injections, all mice received intracardiac injection of luciferase-labeled MDA-MB-231 cells. Three weeks later, tissues were collected for RT-qPCR of luciferase gene using mouse 18S as internal control to quantify metastases. *p < 0.05. Results are presented as mean ± SD. (See also Figure S3.)
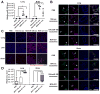
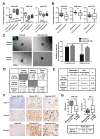
Figure 5. MiR-105 overexpression in poorly metastatic BC cells promotes metastasis in vivo
(A) Luciferase-labeled MCFDCIS/vec or MCFDCIS/miR-105 cells were injected into the No. 4 mammary fat pad of NSG mice (n = 8). BLI at week 6 was shown. *Due to the extensive tumor burden these 3 mice were sacrificed at week 5.5; their images at week 5 were shown. (B) Quantification of metastases in lung and brain. Mice shown in A were sacrificed at week 6 and tissues were subjected to RT-qPCR of luciferase gene using mouse 18S as internal control (n = 8). Results are presented as mean ± SD. *p < 0.05. (C) Representative H&E images of the tumor edges showing local invasiveness. Bar = 50 μm. (D) In vivo vascular permeability determined by the appearance of intravenously injected rhodamine-dextran (red) in various organs. Tissues were collected from mice bearing MCFDCIS/vec or MCFDCIS/miR-105 xenografts (n = 3) that were sacrificed at week 6. Representative images are shown. DAPI (blue): cell nuclei. Bar = 100 μm. (E) Representative images of miR-105 ISH in tissues collected from the two groups. Bar = 50 μm. (F) Collected tissues were subjected to double-label IF for ZO-1 (green) and CD31 (pink). Structures positive for CD31 are indicated by arrowheads. Bar = 100 μm. (See also Figure S4.)
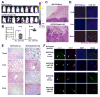
Figure 6. MiR-105 inhibition suppresses metastasis and restores vascular integrity in vivo
(A) Luciferase-labeled MDA-231-HM cells were injected into the No. 4 mammary fat pad of NSG mice. Mice were divided into 3 groups (n = 6) for treatment with PBS, anti-miR-105 compound, or control compound. BLI at week 3 and week 6 were shown. (B) Tumor volume determined in the 3 groups. *p < 0.005 comparing to the other 2 groups. (C) Quantification of metastases in lung and brain. Mice shown in A were sacrificed at week 6 and tissues were subjected to RT-qPCR of luciferase gene using mouse 18S as internal control (n = 6). *p < 0.01. (D) Representative H&E images of the tumor edges showing local invasiveness. Bar = 50 μm. (E) IHC was performed in xenograft tumors using antibodies of Ki-67, cleaved caspase-3 and ZO-1. Representative images are shown. Bar = 50 μm. (F) In vivo vascular permeability indicated by the penetration of rhodamine-dextran (red) into various organs. Tissues were collected from tumor-free NSG mice as well as mice bearing MDA-231-HM tumors that were untreated when sacrificed at week 3 after tumor cell implantation (the pre-metastatic group) or treated as indicated and sacrificed at week 6 (n = 4). Representative images are shown. DAPI (blue): cell nuclei. Bar = 100 μm. (G) Tissues were subjected to double-label IF for ZO-1 (green) and CD31 (pink). Structures positive for CD31 are indicated by arrowheads. Bar = 100 μm. Results are presented as mean ± SD. (See also Figure S5.)
Figure 7. MiR-105 is associated with ZO-1 expression and metastatic progression in BC
(A) MiRNA levels in the sera of tumor-free or MDA-231-HM tumor-bearing mice (Pre-metastasis: serum collected at week 3; Metastasis: serum collected at week 6; n = 5–6) were measured by RT-qPCR and normalized to miR-16. **p > 0.05. (B) Circulating exosomes were isolated from serum samples of stage II–III BC patients. MiRNAs were measured by RT-qPCR, normalized to miR-16, and compared among patients who developed distant metastases during the follow up (n = 16) and those who did not (n = 22). **p > 0.05. (C) Circulating miR-105 in patient serum resulted in a vascular destruction. Vascular structures established from HMVECs that were transfected with anti-miR-105 compound or control compound were treated with human serum from a healthy donor or a BC patient with a high level of circulating miR-105. Representative images of the treated vascular structures were shown (left panel). Inset: structures were stained with CD31 antibody (green) and DAPI (blue). Vascular sprouts per spheroid were counted and graphed (right panel). At least 50 spheroids were counted in each experiment and the experiment was repeated three times. *p < 0.05. (D) Correlation analyses of tumor miR-105, serum (exosomal) miR-105, and ZO-1 levels in BC patients. MiR-105 levels in tumor cells and ZO-1 levels in tumor cells (tumor ZO-1) or tumor-adjacent vascular structures (vascular ZO-1) were determined by ISH and IHC, respectively, and scored as described in Experimental Procedures. Serum (exosomal) miR-105 levels were determined by PCR using miR-16 as a normalizer. Correlation analyses were carried out between two sets of quantified data as indicated. Pearson correlation coefficient (R) and p value are shown. (E) The scores of tumor miR-105, tumor ZO-1, and vascular ZO-1 staining were compared between stage II–III BC patients who developed distant metastases (n = 10) and those who did not (n = 10). Mean and SD of the staining scores in each group are shown. (F) Representative images of miR-105 and ZO-1 staining in tumor and normal breast tissue sections. Vascular structures are indicated by arrowheads. Bar = 100 μm. (G) Levels of tumor miR-105 and ZO-1 determined in a BC tissue array. The ISH or IHC scores were compared between primary tumors with distant or lymph node metastases (n = 15) and those without (n = 60). The correlation between miR-105 and ZO-1 was analyzed among all cases (n = 75). Results are presented as mean ± SD. (See also Figure S6 and Tables S2-5.)
Comment in
- Cancer genetics: Exosomally derived miR-105 destroys tight junctions.
Lokody I. Lokody I. Nat Rev Genet. 2014 Jun;15(6):362. doi: 10.1038/nrg3741. Epub 2014 Apr 29. Nat Rev Genet. 2014. PMID: 24776771 No abstract available. - Genetics: exosomally derived miR-105 destroys tight junctions.
Lokody I. Lokody I. Nat Rev Cancer. 2014 Jun;14(6):386-7. doi: 10.1038/nrc3747. Epub 2014 May 8. Nat Rev Cancer. 2014. PMID: 24804958 No abstract available. - MicroRNA-Based Metastasis Prediction.
Jandial R, Choy C. Jandial R, et al. Neurosurgery. 2015 Aug;77(2):N18. doi: 10.1227/01.neu.0000467296.18386.4b. Neurosurgery. 2015. PMID: 26181789 No abstract available.
Similar articles
- Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins.
Fang JH, Zhang ZJ, Shang LR, Luo YW, Lin YF, Yuan Y, Zhuang SM. Fang JH, et al. Hepatology. 2018 Oct;68(4):1459-1475. doi: 10.1002/hep.29920. Epub 2018 Jul 25. Hepatology. 2018. PMID: 29637568 - A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells.
Png KJ, Halberg N, Yoshida M, Tavazoie SF. Png KJ, et al. Nature. 2011 Dec 14;481(7380):190-4. doi: 10.1038/nature10661. Nature. 2011. PMID: 22170610 - Serum exosomal miR-638 is a prognostic marker of HCC via downregulation of VE-cadherin and ZO-1 of endothelial cells.
Yokota Y, Noda T, Okumura Y, Kobayashi S, Iwagami Y, Yamada D, Tomimaru Y, Akita H, Gotoh K, Takeda Y, Tanemura M, Murakami T, Umeshita K, Doki Y, Eguchi H. Yokota Y, et al. Cancer Sci. 2021 Mar;112(3):1275-1288. doi: 10.1111/cas.14807. Epub 2021 Jan 29. Cancer Sci. 2021. PMID: 33426736 Free PMC article. - The miR-30 family: Versatile players in breast cancer.
Yang SJ, Yang SY, Wang DD, Chen X, Shen HY, Zhang XH, Zhong SL, Tang JH, Zhao JH. Yang SJ, et al. Tumour Biol. 2017 Mar;39(3):1010428317692204. doi: 10.1177/1010428317692204. Tumour Biol. 2017. PMID: 28347244 Review. - Regulation of breast cancer and bone metastasis by microRNAs.
Vimalraj S, Miranda PJ, Ramyakrishna B, Selvamurugan N. Vimalraj S, et al. Dis Markers. 2013;35(5):369-87. doi: 10.1155/2013/451248. Epub 2013 Sep 26. Dis Markers. 2013. PMID: 24191129 Free PMC article. Review.
Cited by
- ncRNAs-mediated overexpression of TET3 predicts unfavorable prognosis and correlates with immunotherapy efficacy in breast cancer.
Liu Y, Wu J, Chen L, Zou J, Yang Q, Tian H, Zheng D, Ji Z, Cai J, Li Z, Chen Y. Liu Y, et al. Heliyon. 2024 Jan 18;10(3):e24855. doi: 10.1016/j.heliyon.2024.e24855. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38318018 Free PMC article. - Clinical relevance of circulating cell-free microRNAs in ovarian cancer.
Nakamura K, Sawada K, Yoshimura A, Kinose Y, Nakatsuka E, Kimura T. Nakamura K, et al. Mol Cancer. 2016 Jun 24;15(1):48. doi: 10.1186/s12943-016-0536-0. Mol Cancer. 2016. PMID: 27343009 Free PMC article. Review. - Increasing the immune activity of exosomes: the effect of miRNA-depleted exosome proteins on activating dendritic cell/cytokine-induced killer cells against pancreatic cancer.
Que RS, Lin C, Ding GP, Wu ZR, Cao LP. Que RS, et al. J Zhejiang Univ Sci B. 2016 May;17(5):352-60. doi: 10.1631/jzus.B1500305. J Zhejiang Univ Sci B. 2016. PMID: 27143262 Free PMC article. - Comparison of serum exosome isolation methods on co-precipitated free microRNAs.
Cheng Y, Qu X, Dong Z, Zeng Q, Ma X, Jia Y, Li R, Jiang X, Williams C, Wang T, Xia W. Cheng Y, et al. PeerJ. 2020 Aug 28;8:e9434. doi: 10.7717/peerj.9434. eCollection 2020. PeerJ. 2020. PMID: 32923177 Free PMC article. - Distinct Cargos of Small Extracellular Vesicles Derived from Hypoxic Cells and Their Effect on Cancer Cells.
Walbrecq G, Margue C, Behrmann I, Kreis S. Walbrecq G, et al. Int J Mol Sci. 2020 Jul 17;21(14):5071. doi: 10.3390/ijms21145071. Int J Mol Sci. 2020. PMID: 32709110 Free PMC article. Review.
References
- Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008. - PMC - PubMed
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. - PubMed
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01CA163586/CA/NCI NIH HHS/United States
- R01 CA166020/CA/NCI NIH HHS/United States
- P30CA33572/CA/NCI NIH HHS/United States
- R01 CA163586/CA/NCI NIH HHS/United States
- R01CA166020/CA/NCI NIH HHS/United States
- P30 CA033572/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases