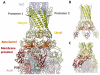Structure of the AcrAB-TolC multidrug efflux pump - PubMed (original) (raw)
. 2014 May 22;509(7501):512-5.
doi: 10.1038/nature13205. Epub 2014 Apr 20.
Affiliations
- PMID: 24747401
- PMCID: PMC4361902
- DOI: 10.1038/nature13205
Structure of the AcrAB-TolC multidrug efflux pump
Dijun Du et al. Nature. 2014.
Abstract
The capacity of numerous bacterial species to tolerate antibiotics and other toxic compounds arises in part from the activity of energy-dependent transporters. In Gram-negative bacteria, many of these transporters form multicomponent 'pumps' that span both inner and outer membranes and are driven energetically by a primary or secondary transporter component. A model system for such a pump is the acridine resistance complex of Escherichia coli. This pump assembly comprises the outer-membrane channel TolC, the secondary transporter AcrB located in the inner membrane, and the periplasmic AcrA, which bridges these two integral membrane proteins. The AcrAB-TolC efflux pump is able to transport vectorially a diverse array of compounds with little chemical similarity, thus conferring resistance to a broad spectrum of antibiotics. Homologous complexes are found in many Gram-negative species, including in animal and plant pathogens. Crystal structures are available for the individual components of the pump and have provided insights into substrate recognition, energy coupling and the transduction of conformational changes associated with the transport process. However, how the subunits are organized in the pump, their stoichiometry and the details of their interactions are not known. Here we present the pseudo-atomic structure of a complete multidrug efflux pump in complex with a modulatory protein partner from E. coli. The model defines the quaternary organization of the pump, identifies key domain interactions, and suggests a cooperative process for channel assembly and opening. These findings illuminate the basis for drug resistance in numerous pathogenic bacterial species.
Figures
Extended Data Figure 1
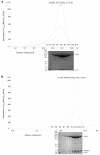
Co-purification of the AcrBZ and AcrBZ/DARPin complexes. A) Gel filtration profile of AcrBZ complex and SDS-PAGE analysis of the peak fractions. B) Gel filtration profile of the AcrBZ/DARPin complex and SDS-PAGE analysis. The proteins were enriched by Immobilised metal affinity chromatography (IMAC, results not shown) before the size exclusion chromatography step. The AcrZ has a heptahistidine tag on the C-terminus as an IMAC affinity tag, and the C-terminal histidines of AcrB have been removed to prevent its binding to the matrix. Standard proteins Thyrogloblin, 669 kDa, and Aldolase, 158 kDa, (Bio-Rad) eluted from the same column at volumes of 11.4 and 14.7 mL respectively. The figure is related to Figure 1 in the main text.
Extended data Figure 2
Validation of the AcrBZ crystal structure. An AcrB protomer taken from the refined structure of the AcrBZ/DARPin complex at 3.3 Å was used in molecular replacement of the 3.7 Å data of the AcrBZ complex (Extended Data Table 1). The model of AcrB without AcrZ was refined with REFMAC jelly-body and reference structure restraints, and a difference map calculated in which AcrZ does not contribute to the phases. The figure shows the positive features of the difference map in the vicinity of AcrZ (green carbon atoms) taken by superposing the AcrB protomers from the AcrBZ/DARPin and AcrBZ (red) structures. The unbiased map shows the presence of AcrZ and indicates that the presence of the DARPin does not disrupt the interactions with AcrB. The model of the AcrBZ/DARPin complex at 3.3 Å was refined without the AcrZ to generate a simulated annealing omit map, which confirmed the location of the AcrZ (not shown). The figure is related to Figure 1 in the main text.
Extended data Figure 3
The AcrBZ complex resembles AcrB-YajC, and AcrBZ interactions involve conserved residues. (A) Superposition of AcrB-YajC (orange and yellow; PDB ID: 2RDD) onto AcrBZ (red and green). The figure supports Figure 1 of the main text. We were not able to identify an interaction between AcrB and C-terminal-histidine-tagged YajC from E. coli, suggesting that the interaction is less avid than the AcrB/AcrZ pairing (data not shown). It is interesting to note that the contacting surface is also conserved in another RND family transporter, SecDF, involved in protein translocation and membrane insertion. It thus seems likely that SecDF might be modulated by a similar helical peptide. Indeed, YajC forms a complex with SecDF, and could play an allosteric role in the process. (B) Sequence conservation of AcrZ. The secondary structure is annotated at the top and asterisks indicate residues that directly contact AcrB. (C) Sequence variation of the surface of AcrB homologues, showing conservation of the surface that contacts AcrZ. The figure on the right includes the bound AcrZ in stick representation (green). The colour spectrum ranges from purple (most conserved) to blue (least conserved). This figure was made with ConSurf .
Extended Data Figure 4
Schematic representations and purification of the fusion proteins used to assemble the efflux pump. (A) The AcrA-AcrB fusion, with two flexible poly(Gly-Ser) linkers. Two C-terminal histidines of AcrB have been removed to prevent binding to the NTA matrix during co-purification. (B) The AcrA-AcrZ-His5 fusion and TolC coexpression construct. The numbers above the bars correspond to the residues of the protein, and due to the restriction site used for cloning, a single glycine residue was inserted after the start codon in both AcrA and AcrZ. The flexible poly(Gly-Ser) linker permits the protomers to manoeuvre. The figure is related to Figure 2 in the main text. (C) Co-purification of TolC with the AcrABZ complexes. SDS-PAGE of the eluate from gel filtration following nickel affinity and size exclusion chromatography purification. The figure is related to Figure 2 in the main text.
Extended data Figure 5
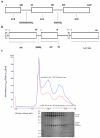
The fusion assemblies can drive efflux in vivo. Drug-transport assays of the AcrAB and AcrAZ fusion proteins in MCΔ_tolC_Δ_acrAB_ strain show that the engineered pump has partial activity for efflux of ethidium bromide (A) and trimethylammonium-diphenylhexatriene (B). The results show representative traces from six biological replicates. For ethidium bromide, initial influx rates of 10.3±0.6, 6.4±0.6 and 3.6±0.4 a.u. (arbitrary units)/min were obtained for the non-expressing control cells, the AcrAB-fusion/AcrAZ-fusion/TolC expressing cells and the wild type AcrAB-TolC expressing cells respectively. For trimethylammonium-diphenylhexatriene, initial influx rates of 187±6, 151±3 and 54±2 a.u. (arbitrary units)/min were obtained for the non-expressing control cells, the AcrAB-fusion/AcrAZ-fusion/TolC expressing cells and the wild-type AcrAB-TolC expressing cells respectively. (C) MIC (Minimum inhibitory concentration) data on antimicrobial susceptibility of Escherichia coli MCΔ_tolC_Δ_acrAB_ cells expressing wild-type AcrA-AcrB-TolC or the fusion proteins with TolC. These data indicate that the fusion of AcrA-AcrZ and the insertion of AcrA into AcrB both diminish the capacity for drug resistance. The plasmids pET21a, pET21a-AcrB or pET21a_-acrB328-polyGS-acrA-polyGS-acrB329_ (AcrAB) together with plasmid pRSFduet-1-acrA-polyGS_-acrZHis5-tolC (AcrAZ + TolC) were transformed into MCΔ_tolC_Δ_acrAB. Cells were tested for their ability to resist increasing concentrations of cytotoxic drugs. As a positive control, cells were transformed with plasmid pBAD_AcrA+AcrB+TolC, which encodes for the native AcrA-AcrB-TolC efflux pump.
Extended data Figure 6
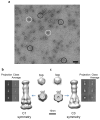
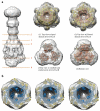
Electron microscopy images and class averages of the AcrABZ-TolC complex. (A) Negative stain EM images of the purified AcrABZ-TolC complex after GraFix treatment. White circles indicate particles with long axis aligned close to the normal to the plane of view; black circles show particles with the long axis parallel to the viewing plane. (B) and (C) Class averages from cryo-EM data and reconstructions with C1 symmetry and with C3 symmetry respectively. The galleries in the side panels show representative two-dimensional class averages of the purified pump. The upper panel shows views perpendicular to the long axis of the drumstick shape, the middle panel shows inclined views, and the lower panel shows views along the long axis. The reconstructed images and cross sections indicate the presence of a six-fold symmetry, which is consistent with 6 AcrA protomers. The pseudo-atomic model has three protomers of AcrB and TolC, and as TolC and AcrB each have an internal structural repeat, they have pseudo six-fold symmetry at low resolution (cut view). Both maps are consistent with each other. This figure is related to Figure 2 in the main text.
Extended Data Figure 7
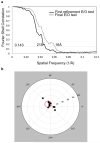
Cryo-EM map resolution estimation and map validation. A) Fourier shell correlation (FSC) of two independently determined cryo-EM maps of the efflux pump assembly after their alignment by Foldhunter. B) Tilt-pair validation of the efflux pump assembly. Each point represents a pair of particles with experimentally known relative tilt. The radius indicates the computationally determined amount of tilt, and the azimuth indicates tilt direction. The red circle denotes particle pairs that cluster around the experimental tilt axis geometry thus validating the map in Figure 2.
Extended data Figure 8
Sections through the EM map. A) Slices 1-4: View of planes normal to the three-fold symmetry axis of the pump. B) Slice 5 of pump assembly showing the opening of TolC periplasmic domain. The same transverse plane through the cryo-EM map is shown for with TolC in the closed state, as seen in the crystal structure of the protein in isolation (left, PDB ID: 1EK9), the partially open structure, made by mutations (middle, PDB ID: 2XMN) and the modelled fully opened structure (right).
Figure 1
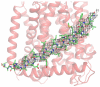
The crystal structure of the inner-membrane protein AcrB in complex with AcrZ. A) Overall view of the AcrB/AcrZ trimer. The subdomains of an AcrB protomer are labelled: PN1, PN2, PC1, PC2, DN and DC; the transmembrane helices are labelled TM1-6, TM7-12 and the linking helix is labelled Iα. AcrB contains a structural repeat and the structural units comprise PN1-PN2-DN-TM1-6 and PC1-PC2-DC-TM7-12. The protein was crystallized with a DARPin, and one is engaged to the AcrB protomer in the crystallographic asymmetric unit (not shown for clarity). B) Electron density map with coefficients 2Fo-Fc overlaid on the model for AcrZ (green sticks), the contour level is 1 σ. AcrB is shown in surface representation, with residues coloured according to their relative hydrophobicity (hydrophobic in red, hydrophilic in white).
Figure 2
Cryo-EM image and a pseudoatomic model of the drug efflux pump. A) A representative raw image of the purified pump. White circles indicate particles with long axis aligned close to the normal to the plane of view; black circles show particles with the long axis parallel to the viewing plane. The scale bar shows 50 nm. B) The reconstructed map and pseudoatomic model. TolC and AcrBZ are homotrimers that engage six protomers of AcrA to form an assembly with a protein mass of 771 kDa. AcrA has a composite structure of four linearly discontinuous domains connected with flexible linkers (see also Figure 3A). C) A slice through the reconstruction and model, which shows the continuous conduit that runs from the AcrB funnel through the TolC porin domain.
Figure 3
Modelled interactions of the efflux pump components. A) Interactions of the AcrA hairpin domain with TolC and the AcrA β-barrel and membrane-proximal domains with AcrB. The lipoyl domains principally interact with each other and make no interactions with AcrB or TolC. Two AcrA protomers in the hexameric ring are shown to illustrate the domain-domain interactions. B) Homohexameric subunit organization in the structure of the AcrA homologue, Actinobacillus actinomycetemcomitans MacA (PDB ID: 4DK0). C) Interactions of E. coli CusA with CusB from the metal-efflux pump.
Similar articles
- In situ structure of the AcrAB-TolC efflux pump at subnanometer resolution.
Chen M, Shi X, Yu Z, Fan G, Serysheva II, Baker ML, Luisi BF, Ludtke SJ, Wang Z. Chen M, et al. Structure. 2022 Jan 6;30(1):107-113.e3. doi: 10.1016/j.str.2021.08.008. Epub 2021 Sep 9. Structure. 2022. PMID: 34506732 Free PMC article. - The C-terminal domain of AcrA is essential for the assembly and function of the multidrug efflux pump AcrAB-TolC.
Ge Q, Yamada Y, Zgurskaya H. Ge Q, et al. J Bacteriol. 2009 Jul;191(13):4365-71. doi: 10.1128/JB.00204-09. Epub 2009 May 1. J Bacteriol. 2009. PMID: 19411330 Free PMC article. - Funnel-like hexameric assembly of the periplasmic adapter protein in the tripartite multidrug efflux pump in gram-negative bacteria.
Xu Y, Lee M, Moeller A, Song S, Yoon BY, Kim HM, Jun SY, Lee K, Ha NC. Xu Y, et al. J Biol Chem. 2011 May 20;286(20):17910-20. doi: 10.1074/jbc.M111.238535. Epub 2011 Mar 29. J Biol Chem. 2011. PMID: 21454662 Free PMC article. - Structures and Efflux Mechanisms of the AcrAB-TolC Pump.
Yu Z, Shi X, Wang Z. Yu Z, et al. Subcell Biochem. 2024;104:1-16. doi: 10.1007/978-3-031-58843-3_1. Subcell Biochem. 2024. PMID: 38963480 Review. - AcrAB and related multidrug efflux pumps of Escherichia coli.
Nikaido H, Zgurskaya HI. Nikaido H, et al. J Mol Microbiol Biotechnol. 2001 Apr;3(2):215-8. J Mol Microbiol Biotechnol. 2001. PMID: 11321576 Review.
Cited by
- Mechanism of coupling drug transport reactions located in two different membranes.
Zgurskaya HI, Weeks JW, Ntreh AT, Nickels LM, Wolloscheck D. Zgurskaya HI, et al. Front Microbiol. 2015 Feb 24;6:100. doi: 10.3389/fmicb.2015.00100. eCollection 2015. Front Microbiol. 2015. PMID: 25759685 Free PMC article. Review. - Structure Determination by Single-Particle Cryo-Electron Microscopy: Only the Sky (and Intrinsic Disorder) is the Limit.
Nwanochie E, Uversky VN. Nwanochie E, et al. Int J Mol Sci. 2019 Aug 27;20(17):4186. doi: 10.3390/ijms20174186. Int J Mol Sci. 2019. PMID: 31461845 Free PMC article. Review. - Contribution of the efflux pump AcrAB-TolC to the tolerance of chlorhexidine and other biocides in Klebsiella spp.
Wand ME, Darby EM, Blair JMA, Sutton JM. Wand ME, et al. J Med Microbiol. 2022 Mar;71(3):001496. doi: 10.1099/jmm.0.001496. J Med Microbiol. 2022. PMID: 35324422 Free PMC article. - RND transporters in the living world.
Nikaido H. Nikaido H. Res Microbiol. 2018 Sep-Oct;169(7-8):363-371. doi: 10.1016/j.resmic.2018.03.001. Epub 2018 Mar 22. Res Microbiol. 2018. PMID: 29577985 Free PMC article. Review. - Antibiotic Resistance Mediated by the MacB ABC Transporter Family: A Structural and Functional Perspective.
Greene NP, Kaplan E, Crow A, Koronakis V. Greene NP, et al. Front Microbiol. 2018 May 28;9:950. doi: 10.3389/fmicb.2018.00950. eCollection 2018. Front Microbiol. 2018. PMID: 29892271 Free PMC article. Review.
References
- Du D, Venter H, Pos KM, Luisi BF. The Machinery and Mechanism of Multidrug Efflux in Gram-negative Bacteria. Microbial Efflux Pumps: Current Research. 2013 Chapter 3.
- Koronakis V, Sharff A, Koronakis E, Luisi BF, Hughes C. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature. 2000;405:914–919. - PubMed
- Murakami S, Nakashima R, Yamashita E, Yamaguchi A. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature. 2002;419:587–593. - PubMed
- Seeger MA, Schiefner A, Eicher T, Verrey F, Diederichs K, Pos KM. Structural asymmetry of AcrB trimer suggests a peristaltic pump mechanism. Science. 2006;313:1295–1298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 094229/WT_/Wellcome Trust/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- 076846/WT_/Wellcome Trust/United Kingdom
- P41GM103832/GM/NIGMS NIH HHS/United States
- P41 GM103832/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases