Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways - PubMed (original) (raw)
Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways
Irving L M H Aye et al. Biol Reprod. 2014 Jun.
Abstract
Obese pregnant women have increased levels of proinflammatory cytokines in maternal circulation and placental tissues. However, the pathways contributing to placental inflammation in obesity are largely unknown. We tested the hypothesis that maternal body mass index (BMI) was associated with elevated proinflammatory cytokines in maternal and fetal circulations and increased activation of placental inflammatory pathways. A total of 60 women of varying pre-/early pregnancy BMI, undergoing delivery by Cesarean section at term, were studied. Maternal and fetal (cord) plasma were collected for analysis of insulin, leptin, IL-1beta, IL-6, IL-8, monocyte chemoattractant protein (MCP) 1, and TNFalpha by multiplex ELISA. Activation of the inflammatory pathways in the placenta was investigated by measuring the phosphorylated and total protein expression of p38-mitogen-activated protein kinase (MAPK), c-Jun-N-terminal kinase (JNK)-MAPK, signal transducer-activated transcription factor (STAT) 3, caspase-1, IL-1beta, IkappaB-alpha protein, and p65 DNA-binding activity. To determine the link between activated placental inflammatory pathways and elevated maternal cytokines, cultured primary human trophoblast (PHT) cells were treated with physiological concentrations of insulin, MCP-1, and TNFalpha, and inflammatory signaling analyzed by Western blot. Maternal BMI was positively correlated with maternal insulin, leptin, MCP-1, and TNFalpha, whereas only fetal leptin was increased with BMI. Placental phosphorylation of p38-MAPK and STAT3, and the expression of IL-1beta protein, were increased with maternal BMI; phosphorylation of p38-MAPK was also correlated with birth weight. In contrast, placental NFkappaB, JNK and caspase-1 signaling, and fetal cytokine levels were unaffected by maternal BMI. In PHT cells, p38-MAPK was activated by MCP-1 and TNFalpha, whereas STAT3 phosphorylation was increased following TNFalpha treatment. Maternal BMI is associated with elevated maternal cytokines and activation of placental p38-MAPK and STAT3 inflammatory pathways, without changes in fetal systemic inflammatory profile. Activation of p38-MAPK by MCP-1 and TNFalpha, and STAT3 by TNFalpha, suggests a link between elevated proinflammatory cytokines in maternal plasma and activation of placental inflammatory pathways. We suggest that inflammatory processes associated with elevated maternal BMI may influence fetal growth by altering placental function.
Keywords: cytokines; innate immune response; obesity.
© 2014 by the Society for the Study of Reproduction, Inc.
Figures
FIG. 1
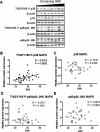
Placental p38-MAPK and JNK-MAPK signaling in relation to maternal BMI. A) Representative Western blots of phosphorylated p38-MAPK (T182/Y180) and JNK-MAPK (T183/Y185) and total p38-MAPK and JNK-MAPK protein expression in homogenates of placentas from pregnancies with varying maternal BMI (n = 32). Scatter plots demonstrate relationship between maternal BMI and placental p38-MAPK (T182/Y180) phosphorylation (B), p38-MAPK expression (C), p46/54 JNK-MAPK (T183/Y185) phosphorylation (D), and p46/54 JNK-MAPK expression (E). Line of best fit indicates significant correlation with maternal BMI.
FIG. 2
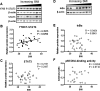
Placental STAT3 and NFκB activity in relation to maternal BMI. Representative Western blots of phosphorylated STAT3 (Y705) and STAT3 (A), and IκBα (D) protein expression in homogenates of placentas from pregnancies with varying maternal BMI (n = 32). Scatter plots demonstrate relationship between maternal BMI and placental STAT3 (Y705) phosphorylation (B), STAT3 expression (C), IκBα expression (E), and nuclear p65 DNA-binding activity (n = 21; F). Line of best fit indicates significant correlation with maternal BMI.
FIG. 3
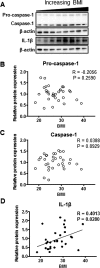
Activation of placental inflammasome complex in relation to maternal BMI. A) Representative Western blots of pro-caspase-1, caspase-1, and IL-1β protein expression in homogenates of placentas from pregnancies with varying maternal BMI (n = 32). Scatter plots demonstrate relationship between maternal BMI and placental pro-caspase-1 (B), caspase-1 (C), and IL-1β (D) expression. Line of best fit indicates significant correlation with maternal BMI.
FIG. 4
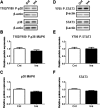
Influence of insulin on p38-MAPK and STAT3 phosphorylation in cultured PHTs. Representative Western blots of phosphorylated p38-MAPK (T182/Y180) and p38 MAPK (A), and phosphorylated STAT3 (Y705) and STAT3 expression (D) in PHT cells treated with insulin (5.8 ng/ml) or vehicle control. Histograms illustrate relative protein expression of phosphorylated p38-MAPK (T182/Y180) (B), p38-MAPK (C), phosphorylated STAT3 (Y705) (E), and STAT3 (F). Mean + SEM, n = 4. Cnt, control; Ins, insulin.
FIG. 5
Influence of MCP-1 and TNFα on p38-MAPK and STAT3 phosphorylation in cultured PHTs. Representative Western blots of phosphorylated p38-MAPK (T182/Y180) and p38 MAPK (A), and phosphorylated STAT3 (Y705) and STAT3 (D) expression in PHT cells treated with MCP-1 (100 pg/ml), TNFα (10 pg/ml), or vehicle control (Cnt). Histograms illustrate relative protein expression of phosphorylated p38-MAPK (T182/Y180) (B), p38-MAPK (C), phosphorylated STAT3 (Y705) (E), and STAT3 (F). Mean + SEM, n = 4. ** P < 0.01, *** P < 0.001 versus control.
Similar articles
- Early growth response protein-1 mediates lipotoxicity-associated placental inflammation: role in maternal obesity.
Saben J, Zhong Y, Gomez-Acevedo H, Thakali KM, Borengasser SJ, Andres A, Shankar K. Saben J, et al. Am J Physiol Endocrinol Metab. 2013 Jul 1;305(1):E1-14. doi: 10.1152/ajpendo.00076.2013. Epub 2013 Apr 30. Am J Physiol Endocrinol Metab. 2013. PMID: 23632636 Free PMC article. Clinical Trial. - Interleukin-1β inhibits insulin signaling and prevents insulin-stimulated system A amino acid transport in primary human trophoblasts.
Aye IL, Jansson T, Powell TL. Aye IL, et al. Mol Cell Endocrinol. 2013 Dec 5;381(1-2):46-55. doi: 10.1016/j.mce.2013.07.013. Epub 2013 Jul 25. Mol Cell Endocrinol. 2013. PMID: 23891856 Free PMC article. - NFκB and JNK/MAPK activation mediates the production of major macrophage- or dendritic cell-recruiting chemokine in human first trimester decidual cells in response to proinflammatory stimuli.
Li M, Wu ZM, Yang H, Huang SJ. Li M, et al. J Clin Endocrinol Metab. 2011 Aug;96(8):2502-11. doi: 10.1210/jc.2011-0055. Epub 2011 Jun 15. J Clin Endocrinol Metab. 2011. PMID: 21677045 Free PMC article. - Placental function in maternal obesity.
Kelly AC, Powell TL, Jansson T. Kelly AC, et al. Clin Sci (Lond). 2020 Apr 30;134(8):961-984. doi: 10.1042/CS20190266. Clin Sci (Lond). 2020. PMID: 32313958 Free PMC article. Review. - Mitogen-activated protein kinases in innate immunity.
Arthur JS, Ley SC. Arthur JS, et al. Nat Rev Immunol. 2013 Sep;13(9):679-92. doi: 10.1038/nri3495. Epub 2013 Aug 19. Nat Rev Immunol. 2013. PMID: 23954936 Review.
Cited by
- Impact of parental obesity on neonatal markers of inflammation and immune response.
Broadney MM, Chahal N, Michels KA, McLain AC, Ghassabian A, Lawrence DA, Yeung EH. Broadney MM, et al. Int J Obes (Lond). 2017 Jan;41(1):30-37. doi: 10.1038/ijo.2016.187. Epub 2016 Oct 26. Int J Obes (Lond). 2017. PMID: 27780976 Free PMC article. - Factors influencing establishment of the ovarian reserve and their effects on fertility.
Monniaux D. Monniaux D. Anim Reprod. 2018 Aug 3;15(Suppl 1):635-647. doi: 10.21451/1984-3143-AR2018-0011. eCollection 2018 Jul-Sep. Anim Reprod. 2018. PMID: 36249842 Free PMC article. - Nitric oxide synthase and VEGF expression in full-term placentas of obese women.
Salvolini E, Vignini A, Sabbatinelli J, Lucarini G, Pompei V, Sartini D, Cester AM, Ciavattini A, Mazzanti L, Emanuelli M. Salvolini E, et al. Histochem Cell Biol. 2019 Dec;152(6):415-422. doi: 10.1007/s00418-019-01819-y. Epub 2019 Sep 24. Histochem Cell Biol. 2019. PMID: 31552486 - Maternal anthropometric measurements and correlation to maternal and fetal outcomes in late pregnancy.
Boucher T, Farmer L, Moretti M, Lakhi NA. Boucher T, et al. Womens Health (Lond). 2022 Jan-Dec;18:17455065221076737. doi: 10.1177/17455065221076737. Womens Health (Lond). 2022. PMID: 35107042 Free PMC article. - Maternal cardiometabolic markers are associated with fetal growth: a secondary exploratory analysis of the LIMIT randomised trial.
O'Brien CM, Louise J, Deussen A, Dodd JM. O'Brien CM, et al. BMC Endocr Disord. 2019 Oct 10;19(1):97. doi: 10.1186/s12902-019-0416-x. BMC Endocr Disord. 2019. PMID: 31601214 Free PMC article. Clinical Trial.
References
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. - PubMed
- Galtier-Dereure F, Boegner C, Bringer J. Obesity and pregnancy: complications and cost. Am J Clin Nutr. 2000;71:1242S–1248S. - PubMed
- Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010;140:387–398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous