Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials - PubMed (original) (raw)
Randomized Controlled Trial
Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials
Carolyn S Calfee et al. Lancet Respir Med. 2014 Aug.
Abstract
Background: Subphenotypes have been identified within heterogeneous diseases such as asthma and breast cancer, with important therapeutic implications. We assessed whether subphenotypes exist within acute respiratory distress syndrome (ARDS), another heterogeneous disorder.
Methods: We used data from two ARDS randomised controlled trials (ARMA trial and ALVEOLI trial), sponsored by the National Heart, Lung, and Blood Institute. We applied latent class modelling to identify subphenotypes using clinical and biological data. We modelled data from both studies independently. We then tested the association of subphenotypes with clinical outcomes in both cohorts and with the response to positive end-expiratory pressure (PEEP) in the ALVEOLI cohort.
Findings: We analysed data for 1022 patients: 473 in the ARMA cohort and 549 in the ALVEOLI cohort. Independent latent class models indicated that a two-class (ie, two subphenotype) model was the best fit for both cohorts. In both cohorts, we identified a hyperinflammatory subphenotype (phenotype 2) that was characterised by higher plasma concentrations of inflammatory biomarkers, a higher prevalence of vasopressor use, lower serum bicarbonate concentrations, and a higher prevalence of sepsis than phenotype 1. Participants in phenotype 2 had higher mortality and fewer ventilator-free days and organ failure-free days in both cohorts than did those in phenotype 1 (p<0·007 for all). In the ALVEOLI cohort, the effects of ventilation strategy (high PEEP vs low PEEP) on mortality, ventilator-free days and organ failure-free days differed by phenotype (p=0·049 for mortality, p=0·018 for ventilator-free days, p=0·003 for organ-failure-free days).
Interpretation: We have identified two subphenotypes within ARDS, one of which is categorised by more severe inflammation, shock, and metabolic acidosis and by worse clinical outcomes. Response to treatment in a randomised trial of PEEP strategies differed on the basis of subphenotype. Identification of ARDS subphenotypes might be useful in selecting patients for future clinical trials.
Funding: National Institutes of Health.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of Interest: Dr. Calfee reports grants from NIH/NHLBI during the conduct of the study; grants from Glaxo Smith Kline, personal fees from Cerus Corp, outside the submitted work. Dr. Delucchi has nothing to disclose. Dr. Thompson reports grants from National Institutes of Health during the conduct of the study. Dr. Parsons has nothing to disclose. Dr. Ware has nothing to disclose. Dr. Matthay reports grants from NHLBI during the conduct of the study; grants from GlaxoSmithKline, other from RocheGenetec, personal fees from Cerus Inc, other from French Society of Intensive Care, outside the submitted work.
Figures
Figure 1
Flow diagram of patient inclusion in the first (ARMA) cohort and second (ALVEOLI) cohort.
Figure 2
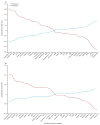
Differences in the standardized values of each variable by phenotype on the y-axis, with the individual continuous variables along the x-axis, for the ARMA cohort (Figure 2A) and the ALVEOLI cohort (Figure 2B). The variables are sorted based on the degree of separation between the classes from maximum positive separation on the left (i.e. Phenotype 2 higher than Phenotype 1) to maximum negative separation on the right (i.e. Phenotype 2 lower than Phenotype 1). Variable standardization, in which all means are scaled to zero and standard deviations to one, is described in the Supplementary Methods; a value of +1 for the standardized variable signifies that the mean value for a given phenotype was one standard deviation higher than the mean value in the cohort as a whole. Abbreviations: IL = interleukin; TNFr1 = tumor necrosis factor receptor-1; PAI-1 = plasminogen activator inhibitor-1; MinVent = total minute ventilation; ICAM-1 = intercellular adhesion molecule-1; MAP = mean airway pressure; VWF = von Willebrand Factor; Creat = creatinine; Resp Rate = respiratory rate; Tbili = total bilirubin; PEEP = positive end expiratory pressure; Temp = temperature in Celsius; Hct = hematocrit; Urine = urine output over prior 24h; SP-D = surfactant protein D; BMI = body mass index; TV = tidal volume; Gluc = glucose; SBP = systolic blood pressure; ProtC = Protein C; Bicarb = bicarbonate; Aspir = aspiration; Pneum = pneumonia.
Figure 3
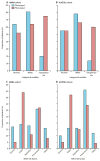
Figure 3A. Differences in categorical variables based on phenotype assignment in the ARMA cohort; p=0.007 for comparison of gender, p<0.0001 for other comparisons. Figure 3B. Differences in ARDS risk factor by phenotype in the ARMA cohort; p<0.0001. Figure 3C. Differences in categorical variables based on phenotype assignment in the ALVEOLI cohort; p=0.96 for comparison of gender, p=0.004 for race, p<0.0001 for vasopressors. Figure 3D. Differences in ARDS risk factor by phenotype in the ALVEOLI cohort; p<0.0001.
Comment in
- Pattern recognition in ARDS: a crucial first step toward personalised treatment.
Reilly JP, Meyer NJ. Reilly JP, et al. Lancet Respir Med. 2014 Aug;2(8):594-5. doi: 10.1016/S2213-2600(14)70116-X. Epub 2014 May 19. Lancet Respir Med. 2014. PMID: 24853586 No abstract available.
Similar articles
- Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial.
Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, Shankar-Hari M, McDowell C, Laffey JG, O'Kane CM, McAuley DF; Irish Critical Care Trials Group. Calfee CS, et al. Lancet Respir Med. 2018 Sep;6(9):691-698. doi: 10.1016/S2213-2600(18)30177-2. Epub 2018 Aug 2. Lancet Respir Med. 2018. PMID: 30078618 Free PMC article. Clinical Trial. - Defining phenotypes and treatment effect heterogeneity to inform acute respiratory distress syndrome and sepsis trials: secondary analyses of three RCTs.
Shankar-Hari M, Santhakumaran S, Prevost AT, Ward JK, Marshall T, Bradley C, Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, McDowell C, Laffey JG, Gordon A, O’Kane CM, McAuley DF. Shankar-Hari M, et al. Southampton (UK): NIHR Journals Library; 2021 Jul. Southampton (UK): NIHR Journals Library; 2021 Jul. PMID: 34347404 Free Books & Documents. Review. - Validation and utility of ARDS subphenotypes identified by machine-learning models using clinical data: an observational, multicohort, retrospective analysis.
Maddali MV, Churpek M, Pham T, Rezoagli E, Zhuo H, Zhao W, He J, Delucchi KL, Wang C, Wickersham N, McNeil JB, Jauregui A, Ke S, Vessel K, Gomez A, Hendrickson CM, Kangelaris KN, Sarma A, Leligdowicz A, Liu KD, Matthay MA, Ware LB, Laffey JG, Bellani G, Calfee CS, Sinha P; LUNG SAFE Investigators and the ESICM Trials Group. Maddali MV, et al. Lancet Respir Med. 2022 Apr;10(4):367-377. doi: 10.1016/S2213-2600(21)00461-6. Epub 2022 Jan 10. Lancet Respir Med. 2022. PMID: 35026177 Free PMC article. - Development and validation of parsimonious algorithms to classify acute respiratory distress syndrome phenotypes: a secondary analysis of randomised controlled trials.
Sinha P, Delucchi KL, McAuley DF, O'Kane CM, Matthay MA, Calfee CS. Sinha P, et al. Lancet Respir Med. 2020 Mar;8(3):247-257. doi: 10.1016/S2213-2600(19)30369-8. Epub 2020 Jan 13. Lancet Respir Med. 2020. PMID: 31948926 Free PMC article. - High versus low positive end-expiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory distress syndrome.
Santa Cruz R, Rojas JI, Nervi R, Heredia R, Ciapponi A. Santa Cruz R, et al. Cochrane Database Syst Rev. 2013 Jun 6;2013(6):CD009098. doi: 10.1002/14651858.CD009098.pub2. Cochrane Database Syst Rev. 2013. PMID: 23740697 Free PMC article. Updated. Review.
Cited by
- Unsupervised Clustering in Neurocritical Care: A Systematic Review.
Tas J, Rass V, Ianosi BA, Heidbreder A, Bergmann M, Helbok R. Tas J, et al. Neurocrit Care. 2024 Nov 19. doi: 10.1007/s12028-024-02140-w. Online ahead of print. Neurocrit Care. 2024. PMID: 39562386 Review. - A randomized control trial evaluating the advice of a physiological-model/digital twin-based decision support system on mechanical ventilation in patients with acute respiratory distress syndrome.
Patel BV, Mumby S, Johnson N, Handslip R, Patel S, Lee T, Andersen MS, Falaschetti E, Adcock IM, McAuley DF, Takata M, Staudinger T, Karbing DS, Jabaudon M, Schellongowski P, Rees SE; DeVENT Study Group. Patel BV, et al. Front Med (Lausanne). 2024 Oct 30;11:1473629. doi: 10.3389/fmed.2024.1473629. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39540041 Free PMC article. - Highlights from the Respiratory Failure and Mechanical Ventilation Conference 2024.
Bianquis C, De Leo G, Morana G, Duarte-Silva M, Nolasco S, Vilde R, Tripipitsiriwat A, Viegas P, Purenkovs M, Duiverman M, Karagiannids C, Fisser C. Bianquis C, et al. Breathe (Sheff). 2024 Nov 12;20(3):240105. doi: 10.1183/20734735.0105-2024. eCollection 2024 Oct. Breathe (Sheff). 2024. PMID: 39534488 Free PMC article. - Heterogeneous Treatment Effects of High-Frequency Oscillatory Ventilation for Acute Respiratory Distress Syndrome: A Post Hoc Analysis of the Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) Trial.
Kobayashi H, Angriman F, Ferguson ND, Adhikari NKJ. Kobayashi H, et al. Crit Care Explor. 2024 Nov 7;6(11):e1178. doi: 10.1097/CCE.0000000000001178. eCollection 2024 Nov. Crit Care Explor. 2024. PMID: 39525347 Free PMC article. Clinical Trial. - Clustering based on renal and inflammatory admission parameters in critically ill patients admitted to the ICU.
Mascle O, Dupuis C, Brailova M, Bonnet B, Mirand A, De Beauchene RC, Philipponnet C, Adda M, Calvet L, Cassagnes L, Henquell C, Sapin V, Evrard B, Souweine B. Mascle O, et al. PLoS One. 2024 Nov 1;19(11):e0307938. doi: 10.1371/journal.pone.0307938. eCollection 2024. PLoS One. 2024. PMID: 39485788 Free PMC article.
References
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967 Aug 12;2(7511):319–23. - PubMed
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994 Mar;149(3 Pt 1):818–24. - PubMed
- Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012 Jun 20;307(23):2526–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01-HR 46056/HR/NHLBI NIH HHS/United States
- N01-HR 46063/HR/NHLBI NIH HHS/United States
- HL090833/HL/NHLBI NIH HHS/United States
- N01-HR 46060/HR/NHLBI NIH HHS/United States
- R01 HL051856/HL/NHLBI NIH HHS/United States
- N01-HR 46057/HR/NHLBI NIH HHS/United States
- N01-HR 46061/HR/NHLBI NIH HHS/United States
- N01-HR 46054/HR/NHLBI NIH HHS/United States
- N01-HR 46059/HR/NHLBI NIH HHS/United States
- N01HR46054/HL/NHLBI NIH HHS/United States
- HL112656/HL/NHLBI NIH HHS/United States
- N01-HR 46055/HR/NHLBI NIH HHS/United States
- R01 HL110969/HL/NHLBI NIH HHS/United States
- HL103836/HL/NHLBI NIH HHS/United States
- N01-HR 46058/HR/NHLBI NIH HHS/United States
- HL 51856/HL/NHLBI NIH HHS/United States
- K24 HL103836/HL/NHLBI NIH HHS/United States
- N01-HR 46064/HR/NHLBI NIH HHS/United States
- R37 HL051856/HL/NHLBI NIH HHS/United States
- 110969/PHS HHS/United States
- R21 HL112656/HL/NHLBI NIH HHS/United States
- K23 HL090833/HL/NHLBI NIH HHS/United States
- N01-HR 46062/HR/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources