Breaking the Gingival Epithelial Barrier: Role of the Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin in Oral Infectious Disease - PubMed (original) (raw)
Breaking the Gingival Epithelial Barrier: Role of the Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin in Oral Infectious Disease
Joseph M DiRienzo. Cells. 2014.
Abstract
The Gram-negative bacterium Aggregatibacter actinomycetemcomitans is part of the HACEK group that causes infective endocarditis, a constituent of the oral flora that promotes some forms of periodontal disease and a member of the family of species that secrete a cytolethal distending toxin (Cdt). The family of bacteria that express the cdt genes participate in diseases that involve the disruption of a mucosal or epithelial layer. In vitro studies have shown that human gingival epithelial cells (HGEC) are native targets of the Cdt that typically induces DNA damage that signals growth arrest at the G2/M interphase of the cell cycle. The gingival epithelium is an early line of defense in the oral cavity against microbial assault. When damaged, bacteria collectively gain entry into the underlying connective tissue where microbial products can affect processes and pathways in infiltrating inflammatory cells culminating in the destruction of the attachment apparatus of the tooth. One approach has been the use of an ex vivo gingival explant model to assess the effects of the Cdt on the morphology and integrity of the tissue. The goal of this review is to provide an overview of these studies and to critically examine the potential contribution of the Cdt to the breakdown of the protective gingival barrier.
Figures
Figure 1
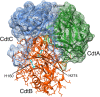
Computer model of the _Aa_Cdt showing the structural relationship of the three subunits: CdtA, CdtB and CdtC. The subunits are depicted as ribbon backbone models using the Protein Data Bank (PDB) file 2F2F, deposited by Yamada et al. [29], and UCSF Chimera 1.8.1. Side chains are shown only in CdtB. The surfaces of CdtA and CdtC are shown as mesh models. Two residues, H160 and H274, in the active site of CdtB are required for toxin activity. Abbreviations: Cdt, cytolethal distending toxin.
Figure 2
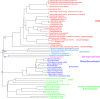
Rooted phylogenetic tree of the deduced amino acid sequences of a representative group of deoxyribonucleases (DNase I), inositol polyphosphate 5-phosphatases and sphingomyelinase. The tree was constructed with PHYLIP 3.6. The lengths of the lines show relative genetic distances.
Figure 3
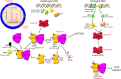
Checkpoint pathway, in response to DNA damage, that leads to growth arrest at the G2/M interphase of the cell cycle in epithelioid cells. The inset illustrates the standard DNA damage checkpoints at G1/S and G2/M in the cell cycle. Cell cycle arrest at G2/M can be measured by flow cytometry or cell sorting. Populations that are arrested at G2/M accumulate cells that have a 4_n_ DNA content.
Figure 4
Human gingival explant (HGX) model of the effects of secreted _Aa_Cdt on tissue obtained from clinically healthy subjects. HGX were untreated or exposed to recombinant wild-type _Aa_Cdt (CdtABC) or native toxin secreted by the bacterium (Cdtsec). Tissue sections were stained with hematoxylin and eosin. Abbreviations: KL, keratinized layer; SL, spinous layer; RP, rete peg; CT, connective tissue. Inset shows the overall histology of the gingival tissues and various types of epithelium (junctional, sulcular and oral) in the upper jaw of a rat. The tissue was decalcified, sectioned and stained with hematoxylin and eosin.
Figure 5
Model of the effects of the _Aa_Cdt on gingival epithelial cell adherens junctions in situ. E-cadherin was detected with a polyclonal antibody conjugated to fluorescein (red fluorescence) in untreated HGX and explants exposed to recombinant _Aa_Cdt. Cell nuclei were stained with DAPI (blue fluorescence). Abbreviations: SL, spinous layer; RP, rete peg; CT, connective tissue.
Similar articles
- Cytolethal distending toxin damages the oral epithelium of gingival explants.
Damek-Poprawa M, Haris M, Volgina A, Korostoff J, DiRienzo JM. Damek-Poprawa M, et al. J Dent Res. 2011 Jul;90(7):874-9. doi: 10.1177/0022034511403743. Epub 2011 Apr 6. J Dent Res. 2011. PMID: 21471326 Free PMC article. - Cell junction remodeling in gingival tissue exposed to a microbial toxin.
Damek-Poprawa M, Korostoff J, Gill R, DiRienzo JM. Damek-Poprawa M, et al. J Dent Res. 2013 Jun;92(6):518-23. doi: 10.1177/0022034513486807. Epub 2013 Apr 10. J Dent Res. 2013. PMID: 23576426 Free PMC article. - Resistance of human periodontal ligament fibroblasts to the cytolethal distending toxin of Actinobacillus actinomycetemcomitans.
Kanno F, Korostoff J, Volgina A, DiRienzo JM. Kanno F, et al. J Periodontol. 2005 Jul;76(7):1189-201. doi: 10.1902/jop.2005.76.7.1189. J Periodontol. 2005. PMID: 16018764 Free PMC article. - Aggregatibacter actinomycetemcomitans, a potent immunoregulator of the periodontal host defense system and alveolar bone homeostasis.
Herbert BA, Novince CM, Kirkwood KL. Herbert BA, et al. Mol Oral Microbiol. 2016 Jun;31(3):207-27. doi: 10.1111/omi.12119. Epub 2015 Sep 22. Mol Oral Microbiol. 2016. PMID: 26197893 Free PMC article. Review. - Impact of CDT Toxin on Human Diseases.
Faïs T, Delmas J, Serres A, Bonnet R, Dalmasso G. Faïs T, et al. Toxins (Basel). 2016 Jul 15;8(7):220. doi: 10.3390/toxins8070220. Toxins (Basel). 2016. PMID: 27429000 Free PMC article. Review.
Cited by
- Periodontal Disease: The Good, The Bad, and The Unknown.
Sedghi LM, Bacino M, Kapila YL. Sedghi LM, et al. Front Cell Infect Microbiol. 2021 Dec 7;11:766944. doi: 10.3389/fcimb.2021.766944. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34950607 Free PMC article. Review. - Genome-Wide Identification of Host Genes Required for Toxicity of Bacterial Cytolethal Distending Toxin in a Yeast Model.
Denmongkholchai S, Katare P, Choochuay S, Thanyasrisung P, Tsuruda K, Sugai M, Mongkolsuk S, Matangkasombut O. Denmongkholchai S, et al. Front Microbiol. 2019 Apr 26;10:890. doi: 10.3389/fmicb.2019.00890. eCollection 2019. Front Microbiol. 2019. PMID: 31080443 Free PMC article. - Interactions between the Aggregatibacter actinomycetemcomitans secretin HofQ and host cytokines indicate a link between natural competence and interleukin-8 uptake.
Ahlstrand T, Torittu A, Elovaara H, Välimaa H, Pöllänen MT, Kasvandik S, Högbom M, Ihalin R. Ahlstrand T, et al. Virulence. 2018;9(1):1205-1223. doi: 10.1080/21505594.2018.1499378. Virulence. 2018. PMID: 30088437 Free PMC article. - Tools of Aggregatibacter actinomycetemcomitans to Evade the Host Response.
Oscarsson J, Claesson R, Lindholm M, Höglund Åberg C, Johansson A. Oscarsson J, et al. J Clin Med. 2019 Jul 22;8(7):1079. doi: 10.3390/jcm8071079. J Clin Med. 2019. PMID: 31336649 Free PMC article. Review. - Periodontal Disease and Senescent Cells: New Players for an Old Oral Health Problem?
Aquino-Martinez R, Khosla S, Farr JN, Monroe DG. Aquino-Martinez R, et al. Int J Mol Sci. 2020 Oct 9;21(20):7441. doi: 10.3390/ijms21207441. Int J Mol Sci. 2020. PMID: 33050175 Free PMC article. Review.
References
- Norskov-Lauritsen N., Kilian M. Reclassification of Actinobacillus actinomycetemcomitans, Haemophilus aphrophilus, Haemophilus paraphrophilus; Haemophilus segnis as Aggregatibacter actinomycetemcomitans gen. nov., comb. nov., Aggregatibacter aphrophilus comb. nov. and Aggregatibacter segnis comb. nov., and emended description of Aggregatibacter aphrophilus to include V factor-dependent and V factor-independent isolates. Int. J. Syst. Evol. Microbiol. 2006;56:2135–2146. doi: 10.1099/ijs.0.64207-0. - DOI - PubMed
- Feder H.M.J., Roberts J.C., Salazar J., Leopold H.B., Toro-Salazar O. HACEK endocarditis in infants and children: Two cases and a literature review. Pediatr. Infect. Dis. J. 2003;22:557–562. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources